A century ago, the German physicists Otto Stern and Walther Gerlach carried out an experiment that gave an important credibility boost to the new-fangled notion of quantum mechanics. But as Hamish Johnston discovers, their now-famous experiment succeeded even if the physics on which it was based wasn’t quite right
Despite its counter-intuitive weirdness, quantum mechanics ranks as one of the most successful scientific theories of all time. Apart from forming the bedrock of our understanding of atoms and subatomic particles, it has spawned a host of technologies from the laser and transistor to quantum cryptography and quantum computers. But quantum mechanics wasn’t always held in such high regard.
At the start of the 20th century, when the subject was just getting off the ground, many scientists were sceptical of this new-fangled theory. Among the doubters was Otto Stern, who, along with fellow German physicist Walther Gerlach, devised a now-famous experiment to disprove the theory.
Carried out 100 years ago, the experiment involved the two physicists using beams of atoms to test a seemingly bizarre consequence of quantum mechanics known as the “space quantization of angular momentum”. As it happens, their initial interpretation of the experiment proved to be wrong. But their work spurred the development of quantum theory and today the “Stern–Gerlach experiment” is considered a classic of modern physics.
The Stern–Gerlach experiment is at the centre of the venerable conceptual puzzles of quantum mechanics – from the uncertainty principle to entanglement
Bretislav Friedrich, Fritz Haber Institute, Berlin
“It’s at the centre of the venerable conceptual puzzles of quantum mechanics – from the uncertainty principle to entanglement,” says Bretislav Friedrich, a physicist from the Fritz Haber Institute in Berlin who has written extensively about the experiment and Stern and Gerlach’s lives. The experiment, he says, was greeted with “pure astonishment” in 1922 – and it still astonishes physicists today.
Quantum weirdness
Born in 1888 in the Prussian city of Sohrau (now Żory in Poland), Stern was a physical chemist by training, who did a PhD at the University of Breslau on the osmotic pressure of solutions of carbon dioxide. In 1912 he moved to the Charles-Ferdinand University in Prague, attracted by Albert Einstein, who was based there at the time. By attending Einstein’s lecturers in Prague – and later at the ETH Zurich where both moved the following year – Stern quickly became exposed to the early ideas of quantum mechanics.

These ideas included Niels Bohr’s early model of the atom, which was the centrepiece of what is now called the “old quantum theory”. Familiar today as a basic representation of an atom, the Bohr model describes an atom as a dense, positively charged nucleus orbited by negatively charged electrons.
According to classical physics, such electrons should radiate energy and spiral into the nucleus in a matter of picoseconds. As that does not happen in reality, Bohr got around this problem by restricting the electrons to specific atomic orbits, more commonly referred to as orbitals.
Orbital quantization enabled Bohr to explain a phenomenon that had puzzled physicists and chemists for decades – the fact that atoms only absorb and emit light at a discrete set of optical wavelengths. Bohr’s model initially seemed like the right idea, as it allowed him to reproduce a formula for these wavelengths that had been derived in 1888 by the Swedish physicist Johannes Rydberg. The Rydberg formula had given the wavelengths in terms of a series of integers, which we now understand to be the principal quantum numbers of the atomic orbitals.

Quantum theory: weird and wonderful
But as Bohr’s model was studied and honed by leading physicists of the day, something odd became apparent. As a consequence of orbital quantization, it transpired that the component of an electron’s orbital angular momentum along a specific direction must also be quantized. In particular, according to Bohr’s model, an electron in the lowest energy orbital should have only two values of angular momentum along any arbitrary direction. These values would point in opposite directions in space, with no intermediate values permitted.
Known as space quantization, this phenomenon was viewed as even more bizarre than orbital quantization. In fact, Stern was so sceptical of the Bohr model that he vowed to quit physics if it proved to be correct. In 1914, after Stern parted company with Einstein and joined the brand-new University of Frankfurt, a practical opportunity arose for him to put space quantization to the test. Stern realized that if an electron’s orbital angular momentum showed space quantization, then so too would the magnetic moment of an atom.
Testing for space quantization
Stern’s work was initially disrupted by the First World War, when he served in the German army on the Russian front. But on his return to Frankfurt, Stern began experiments on beams of atoms, which had become possible thanks to the invention of the mercury-diffusion vacuum pump by the German physicist Wolfgang Gaede in 1915. This device allowed researchers to create high-vacuum conditions for the first time so that atoms could travel the length of an experimental apparatus without scattering from air molecules.
In 1920 Stern was joined in Frankfurt by Gerlach, who – like Stern – had also served in the First World War. Gerlach had become involved in atomic-beam experiments through his interest in the properties of atoms in magnetic solids. In particular, Gerlach wanted to see if atoms have magnetic moments and had begun thinking about an experiment involving a beam of bismuth atoms travelling through a region with an inhomogeneous magnetic field.

If an atom has a dipole magnetic moment, Gerlach reasoned, it will experience a torque due to the magnetic field and will therefore rotate. But if the magnetic field is not uniform, the force at one end of a dipole will be stronger than the torque at the opposite end. That will lead to a net force on the atom, which will deflect as its flies through the inhomogeneous magnetic field – with the size of the deflection revealing the magnitude of the atom’s magnetic moment.
In 1921 Stern realized that such an experiment would be a great way to test for space quantization. If atoms have magnetic moments that can point in any direction (as classical physics would suggest) then the beam of atoms would broaden continuously as it passes through the inhomogeneous magnetic field. However, if the magnetic moments of the atom are space quantized – pointing in opposite directions (up and down) along the inhomogeneous field – then the beam of atoms would be split in two.
As a result, the up and down atoms would be deflected in opposite directions, providing clear evidence for space quantization. So confident was Stern of his idea that he published a paper in the journal Zeitschrift für Physik (7 249) that presented meticulous calculations describing how it could be done using a beam of silver atoms. Gerlach was convinced and started to build his apparatus in 1921. Stern (a theorist) and Gerlach (an experimentalist) proved an effective combination.
Experimental breakthrough
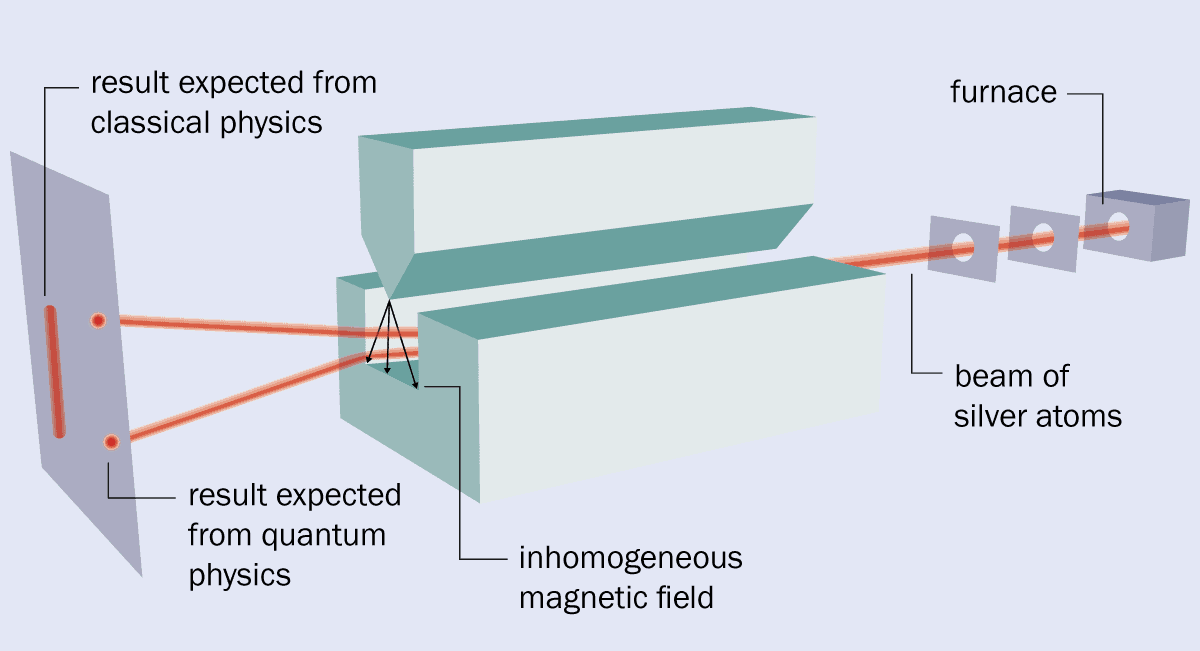
In the original version of the Stern–Gerlach experiment, the two physicists vapourized silver in an oven and allowed some atoms to escape through a hole (see box 1). They then sent the atoms through a pair of collimators, which created a beam that travelled between the two pole pieces of an electromagnet. These pieces provided a magnetic field with the required high level of inhomogeneity because one had a groove cut into it, while the other had a sharp, knife-like edge and was held above the groove.
After passing through the magnet, the beam struck a detector plate where the presence of silver could be revealed by a chemical development process similar to that used in photography. But despite its simplicity, the experiment was fiendishly difficult to undertake.
The apparatus was small – about the size of a fountain pen – and had to be kept under high vacuum using two of Gaede’s diffusion pumps. In fact, the apparatus often broke, making it difficult to achieve the long run time needed to accumulate enough silver on the detector plate to create a visible image.
1 The original set-up
What’s more, the Stern–Gerlach experiment was expensive, made worse by the hyperinflation that was rampaging through post-war Germany. Money and donated equipment had to be secured from a range of sources including the Physikalischer Verein Frankfurt (Frankfurt Physics Society), ticket sales from popular lectures by Max Born, and donations from Einstein and Henry Goldman – an American banker and son of the co-founder of the financial firm Goldman–Sachs.
At first, Stern and Gerlach only saw their beam broadening when the inhomogeneous magnetic field was switched on. This was not the splitting predicted by quantum mechanics, but was an important achievement in its own right, being the first experimental evidence for atoms having magnetic moments. After Stern left Frankfurt in late 1921 to take up a professorship of theoretical physics at the University of Rostock, Gerlach realized that he could improve the measurement by replacing the round holes in the collimators with slits, which boosted the number of atoms in the beam.
And so, working alone one February night in 1922, Gerlach finally saw the beam splitting that had been predicted. He immediately sent a telegram to Stern saying: “Bohr is right after all”. Gerlach also created a postcard showing the split beam and sent it to Bohr, congratulating him for creating his model of the atom. Their paper presenting the results, published in December 1922 (Zeit. Phys. 9 349), provided the first experimental evidence for space quantization in a magnetic field – and thereby crucial evidence for quantum theory.
A new spin on things
The Stern–Gerlach experiment caused an immediate stir in the physics community, with Wolfgang Pauli, for example, quipping that “This should convert even the non-believer Stern”. However, the explanation for the observed beam splitting using Bohr’s model was short-lived.
Other physicists, including Pauli and Paul Dirac, soon realized that the electron has an intrinsic angular momentum or spin – something that was not included Bohr’s model. What’s more, Bohr had been wrong to predict that the ground state of the silver atom has orbital angular momentum; it does not.
Today we know that the splitting that Stern and Gerlach saw in their experiment is actually caused by the spin of silver’s unpaired electron. Indeed, the Stern–Gerlach experiment is now interpreted as evidence of electron spin, rather than as proof of space quantization.
As so often in physics, even supposedly “wrong” results can still lead to progress. What’s more, the work opened the door to a huge variety of other findings, including showing the momentum transfer that occurs if an atom emits or absorbs a photon.
Stern went on to carry out the world’s first matter-wave experiments with atoms when he scattered beams of atoms off the surfaces of crystals, which confirmed the principle of wave-particle duality. Later, in 1933, he measured the magnetic dipole moment of the proton using a set-up similar to the Stern–Gerlach experiment. He found it to be larger than expected, which suggested that the proton is not a point-like particle – as had been assumed at the time – but rather has internal structure. This discovery led Stern to win the Nobel Prize for Physics in 1943.
By that time, Stern – who was Jewish by birth – was based at the Carnegie Institute of Technology in Pittsburgh in the US, having fled Germany in 1933 as Nazi repression of the Jews intensified. While at Carnegie, he extended his work on the magnetic dipole moment of the proton to its heavier cousin, the deuteron. Stern’s lab in Pittsburgh also confirmed Maxwell–Boltzmann velocity distribution of particles in an ideal gas. He died in the US in 1969.
As for Gerlach, he went on to measure the magnetic moment of atomic bismuth and several other metals using the atomic beam technique. He also did experiments on the radiation pressure of light and continued his interest in magnetism and condensed-matter physics. His career later took him to the universities of Tübingen and Munich. Despite being nominated 31 times between 1924 and 1944, Gerlach missed out on a Nobel prize.
[The pair benefitted from] Stern’s ideas on the one hand and Gerlach’s realism and skills in the lab as well as a – sometimes stubborn – determination to make things work
Bretislav Friedrich, Fritz Haber Institute, Berlin
Like Stern, Gerlach was impacted by Nazism – but in a very different way. Although he never joined the Nazi party and rejected the idea of “Jewish science”, Gerlach headed Germany’s nuclear research programme in the final years of the Second World War. He ended up being interned by the Allied Forces at Farm Hall in England along with nine other German physicists suspected of being involved in Germany’s nuclear weapons programme including Max von Laue and Werner Heisenberg.

The quantum heretics
After the war, Gerlach returned to academic research, spending the bulk of his career back at Munich where he played important roles in rebuilding German science. In 1957, he, von Laue and Heisenberg were part of a group of 18 leading German physicists who signed the Göttingen Manifesto, which rejected a proposal by the then chancellor Konrad Adenauer to arm Germany with nuclear weapons. Gerlach died aged 90 in 1979.
Lasting impact
Apart from shaping the course of modern science, the Stern–Gerlach experiment has also had a huge practical impact. Indeed, Stern is widely viewed as one of the founders of experimental atomic, molecular and nuclear physics, having shown how molecular beams can be used to quantitatively study matter without resorting to spectroscopy. “Sorting states via space quantization is ubiquitous,” says Friedrich, with nuclear magnetic resonance and magnetic-resonance imaging being the most direct descendants of the classic experiment.
Friedrich also credits the Stern–Gerlach experiment for introducing principles that influenced other areas of science, such as the development of the maser and laser. And although Gerlach missed out on a Nobel prize, Friedrich says his recruitment by Stern was a “stroke of luck” for atomic physics, with the pair having exceptional complementary talents. “[They benefitted from] Stern’s ideas on the one hand and Gerlach’s realism and skills in the lab as well as a – sometimes stubborn – determination to make things work.”
- For more on the Stern–Gerlach experiment, see Molecular Beams in Physics and Chemistry: From Otto Stern’s Pioneering Exploits to Present-Day Feats edited by Bretislav Friedrich and Horst Schmidt-Böcking (2022 Springer).



