The exact nature of the reactions that underpin heterogeneous catalysis remains an important question in fundamental chemistry research, with implications for many industrial applications. A group of researchers in the Netherlands led by Ludo Juurlink at Leiden University have laid to rest a long debate on the exact mechanism of molecular reactions at surface defects.
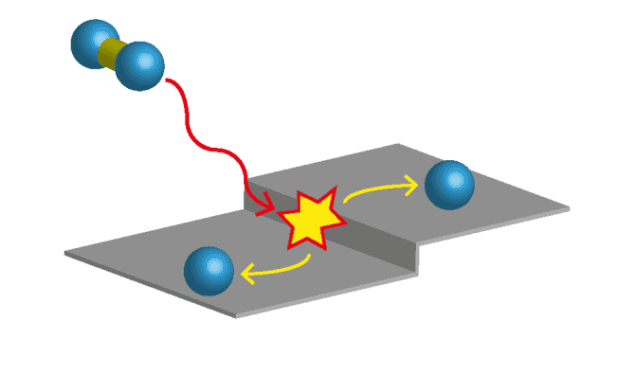
Heterogeneous catalysis describes a set of reactions in which the reactants and catalysts differ in phase (e.g. gases reacting on solids), the most well studied of which is hydrogen gas molecule (H2) dissociation at platinum (Pt) surfaces. But scientists have not been able to agree on the role of the catalyst surface in such reactions. Indeed, two competing models have been developed in recent years to describe the underlying reaction mechanism. The first suggests that molecular H2 physisorbs to the Pt as a mobile reaction intermediate and diffuses freely along the surface until it dissociates at a defect site. The second model does away with mobile precursors and, instead, suggests that H2 gas directly dissociates upon impact with defects on the Pt surface.
In order to investigate this reaction in detail, the researchers designed a curved Pt crystal with highly controlled local concentrations of two common Pt defects, which are step sites and are defined by the lattice plane of the crystal surface: {100} (A-type) and {110} (B-type). Using a home-built supersonic incident molecular beam, they evaluated the initial sticking probability of H2 at regions of varying defect type and density with high precision by measuring the drop in background pressure as beams of molecules adsorb to the sample (known as the King and Wells method).
H2 dissociates by direct impact at defect sites
Comparing the adsorption behaviour of molecular H2 at high and low incident energies, as well as at high and low temperatures, the researchers were able to show that their results were inconsistent with the first model proposed. Indeed, they showed that high surface temperature increased or left unchanged the overall adsorption of H2 onto Pt. In contrast the first model predicts that at a higher temperature, physisorbed H2 will have a shorter residence time on the Pt surface, giving it less time to find a defect and dissociate, instead returning to the gas phase.

Hydrogen dissociation measurement puts theory at odds with experiment
Furthermore, model 1 relies on the assumption that the surface density of defects dominates adsorption. The authors, however, again proved this model wrong by showing that H2 adsorption can be independent of defect type and density at higher incident beam energies.
Finally, the researchers showed that at lower incident beam energies there was a significant increase in adsorption at B-type defects as compared with A-type defects. Model 1 does not take into account site-specific reactivities but only the overall surface density of defects hence model 2 again proves more accurate. These results greatly improve our understanding of defect-mediated reactions and present benchmarks for the future design of high energy surfaces for catalytic applications.
Full details of the research are reported in Science.