If the dissolvable microneedle patches used to deliver vaccines could also implant an invisible record of the inoculation under the skin, a person’s vaccination history could be known with certainty. With this aim, researchers at Massachusetts Institute of Technology have developed a microneedle patch containing a nanocrystal dye that creates near-infrared-emitting dots under the skin. The emitted light is then detected by a specially modified smartphone (Sci. Transl. Med. 10.1126/scitranslmed.aay7162).
The microneedle patches can be customized to imprint different patterns that correspond to the type of vaccine, the date administered, and the vaccine’s manufacturer and lot number. This technique could reduce problems associated with lack of standardized vaccination record-keeping in many regions of the world.
The team’s goal was to develop a robust, inexpensive and easy-to-use platform that could be applied without difficulty in low-resource settings. For their proof-of-concept study, the researchers developed a robust dye that’s resistant to photobleaching, a technique to encapsulate the dye, and a microneedle design to optimize delivery under the skin.
The dye ultimately selected by the team was a colloidal quantum dot (QD) formulation called S10C5H. This formulation retained 13% of its fluorescent signal after exposure to five years of simulated sunlight through pigmented skin samples at sevenfold the intensity of the sun. To improve biocompatibility, the QDs were encapsulated in PMMA microspheres.
To detect signals from the QDs, the researchers adapted an inexpensive smartphone to have near-infrared imaging capability. Modifications included removal of the standard short-pass filter and addition of 850 nm long-pass filters to block both environmental light and light from LED illumination.

Lead authors Kevin McHugh and Lihong Jing and colleagues analysed 50 microneedle shapes to identify one that ensured deep penetration of a high volume of QDs into a permanent layer of skin, and which was highly resistant to mechanical failure. They selected a microneedle with a height of 1500 µm, a diameter of 300 µm and a half-cylindrical/half-tapered design. This needle could pierce the skin deeply enough without bending or fracturing.
The robustness requirement of the new microneedles differed considerably from those developed to deliver vaccines, which only need to break the skin’s water barriers. Additionally, the patch containing the microneedles must be designed to not cause excessive pain and have sufficient spacing for each needle to act independently.
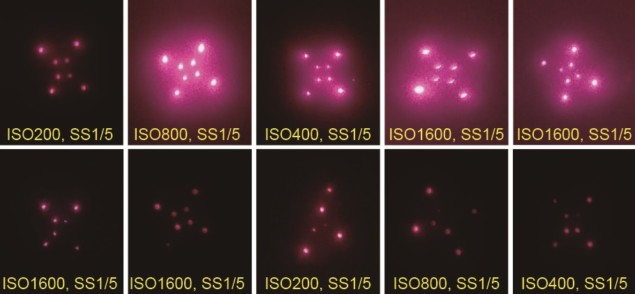
The researchers tested the patches on Wistar rats, administering QD-containing microneedle patches in three distinct patterns on their rear flanks and imaging the animals for nine months. The 120 needle markings were visible on the day of administration, declining to 92% visibility after 24 weeks. Nine months after administration, the signal was 29-fold higher than background signal.
The team also used a machine learning system (AlexNet) to automatically classify each pattern for 210 images acquired biweekly over 30 weeks. Classification accuracy remained above 98.4% in this time.
“Because there was no trend toward lower machine learning classification probability at three, six and nine months, it appears that the patterns are stable after an acute period of signal loss,” the researchers note. The machine learning system also was valuable when markings were missing, dim or imaged at an unusual angle.
Transferring this technology to the clinic will require additional steps including preclinical safety and toxicology studies, manufacturing scale-up and first-in-human studies. If vaccine information could be co-delivered with the vaccine, instead of consecutively, this could reduce costs and eliminate potential errors in failing to administer two patches. However, it will be necessary to ensure that the PMMA-encapsulated QDs do not interfere with the robust immune response generated by intradermal antigen delivery.
“Ultimately, we believe that this invisible, ‘on-body’ technology opens up new avenues for decentralized data storage and biosensing applications that could influence the way medical care is provided, especially in the developing world,” the researchers conclude. They are currently working to develop a dye that will be visible for longer than 10 years.