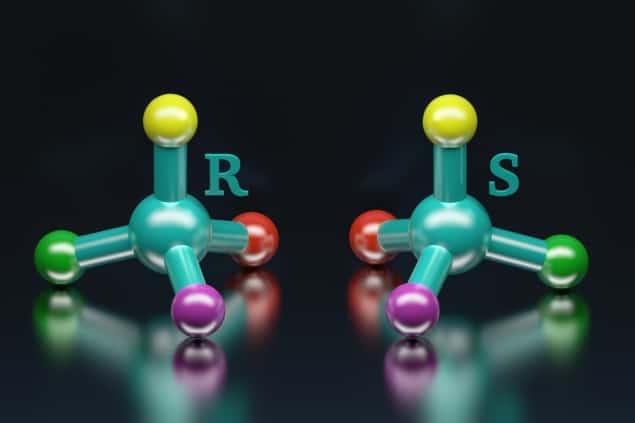
Like the imbalance of matter and antimatter, the emergence of life based on molecules with a like chirality as opposed to their mirror opposites has long puzzled scientists. Chiral molecules, like your hands, cannot be superposed, but in the absence of some sort of chiral catalyst or source, the products of all chemical reactions will have roughly equal amounts of “left-handed” and “right-handed” molecules. So how come almost all the amino acids found in the proteins of living organisms are left-handed and all the sugars are right-handed? According to measurements and analysis recently reported in Nature Chemistry observations of the autocatalytic “Soai” reactions that had looked so promising won’t provide a definitive answer.
Electroweak parity violation and autocatalysis
Chirality is common among organic molecules because of carbon’s four outermost bonding electrons, which gears it towards tetrahedral molecular structures. The one-handedness of biomolecules is more of a mystery. One of the candidate theories hinges on the idea of “parity violation” in interactions of the “electroweak” force, which is one of the four fundamental interactions (alongside the electromagnetic, gravitational and strong forces), and governs radioactive decay of atoms into more stable isotopes.
For most interactions “parity symmetry” is conserved so if you flip the spatial coordinates it makes no fundamental difference to the interaction. However, the suggestion was that the electroweak force violated this parity symmetry so that there would be an energy difference – albeit potentially very small – between a chemical reaction producing one chiral molecule over its mirror image. The parity violation energy difference (PVED) hypothesis gained momentum when Chien-Shung Wu at Columbia University in the US alongside researchers at the Low Temperature Group of the US National Bureau of Standards, and later Richard Garwin, Leon Lederman and Marcel Weinrich, also at Columbia University, reported observations of this imparity in papers published in 1957.
PVED suggested how the product of a reaction might be biased towards one chirality over another but not the emergence of homochirality. However reports of an autocatalytic reaction by Kenso Soai and colleagues at the Science University of Tokyo in Japan in 1995 provided a possible mechanism for homochirality emerging from an initial imbalance. The “Soai reaction” provided a model for a chiral product that then catalysed the reaction to produce more molecules with the same chirality, so that an initial chirality imbalance would snowball.
The catch is that in the absence of some chiral source to tip the reaction into the chirality amplifying regime, the product over the whole system will still be equal parts of both chirality. However Soai also worked out that if the reaction involved isotopically chiral molecules (chiral only by virtue of differences in chemical isotope in the molecule such as 12C versus 13C) then this would lead to the formation of two different complexes in the reaction pathway with slightly differing stability. This stability difference could provide the necessary chiral source to tip the balance. Intrigued, Donna Blackmond and her team at Scripps Research Institute in the US have been working on various aspects of the Soai reaction for nearly 20 years. Alongside Neil Hawbaker at Scripps Research Institute, she has been taking the imbalance down to very low magnitudes to look for the stability difference, and establish how big the stability difference needs to be.

Testing the numbers
To quantify the stability or energy difference the researchers studied the Soai reaction. They combined an aldehyde and di-isopropyl zinc with an isotopically chiral alcohol as an initiator for the first reaction in the sequence. An emerging excess of one chirality of the molecule (also known as an enantiomer) over the other was taken as evidence of chiral symmetry breaking. As a control they also measured the outcome of experiments using an achiral alcohol initiator. From their experiments they concluded that an enantiomeric excess of between 0.1% and 1% in the isotopically chiral initiator was needed to tip the reaction into the chirality amplifying regime.
Next, they turned to theory to calculate the stability of complexes formed in the reaction from the parameters measured in their experiments. Calculating the stochastic outcome of the reaction from a large number of simulations indicated that the resulting enantiomeric excess required to break the chiral symmetry of the reaction was just 3.5 × 10-7. As Hawbaker and Blackmond put it in their report, “a direct experimental measurement of the threshold for symmetry breaking under our conditions would involve an absolute excess enantiomer concentration roughly equivalent to 20 mg of the autocatalyst in an Olympic-sized swimming pool.”
As for the calculated stability difference needed to give a bias towards one type of enantiomer that exceeds stochastic fluctuations, Blackmond and Hawbaker’s calculations give this a value of 0.0002%, and the energy required somewhere between 1.5 × 10−7 and 1.5 × 10−8 kJ mol−1 – five to seven orders of magnitude greater than the best estimates of what the energy difference from electroweak parity violation might be.
While models exist that allow chirality symmetry breaking to occur in autocatalytic reactions as a result of parity violation energy differences much smaller than the energy required, as Blackmond tells Physics World, “It certainly suggests that we would not be able to measure such an effect on such a reaction as the Soai reaction, in today’s chiral world.” These results add to other “inherent challenges” to the hypothesis, such as the absence of any known autocatalytic asymmetric reaction that could occur in the conditions of prebiotic Earth.

Chirality measurements speed up
The result did not greatly surprise Blackmond. “My feeling is that we have not yet found a plausible phenomenon – either a prebiotically relevant autocatalytic reaction or some other chemical or physical process – through which PVED would be sufficiently “symmetry breaking” to allow the subsequent asymmetric amplification that must occur to reach significant levels of enantioenrichment,” she tells Physics World. Her own research will continue trying to bring aspects of chirality into research on prebiotic chemistry carried out in the groups at Scripps Research Institute and those of their collaborators.
Full details are available in Nature Chemistry.