Is there something inherently quantum about the highly efficient natural process that is photosynthesis, or are researchers barking up the wrong tree? Philip Ball investigates the debate
The quirks of quantum physics are something you might expect to find under exotic conditions in a laboratory, but not in a meadow. Yet in recent years, a blossoming idea called quantum biology proposes that life’s molecular mechanisms deploy some of those notoriously counterintuitive behaviours. Ten years ago, researchers reported evidence that photosynthesis – the process by which green plants and some bacteria turn sunlight into chemical energy – gains light-harvesting efficiency by exploiting the phenomenon of “quantum coherence”. This involves the superpositions of electronic quantum states, which seem able to explore many energy-transmitting pathways at once. If so, quantum mechanics is assisting the fundamental energetic process that drives all life on the surface of the Earth.
It was a remarkable claim. But was it true? The question has been hotly debated over the past decade. Early talk of “quantum coherence” in photosynthesis being akin to that in quantum computers, where it underpins the faster and more efficient computation that those devices achieve, has now largely evaporated in favour of a more nuanced picture. And some researchers insist that such coherence plays no useful role in photosynthesis at all.
On the one hand, Greg Engel of the University of Chicago, who was involved in the initial work suggesting quantum coherence as a new design principle of nature, says that “the general notion that the language and mathematics of quantum information, including coherence, can be used to understand photosynthetic dynamics in ultrafast spectroscopy experiments seems to be growing in acceptance”. But biophysical chemist Sebastian Westenhoff of the University of Gothenburg in Sweden says that ever more scientists in the field regard the earlier work as a misinterpretation, and that “there is no such thing as quantum coherence in [natural] photosynthesis”. So what’s the argument all about?
Get the green light
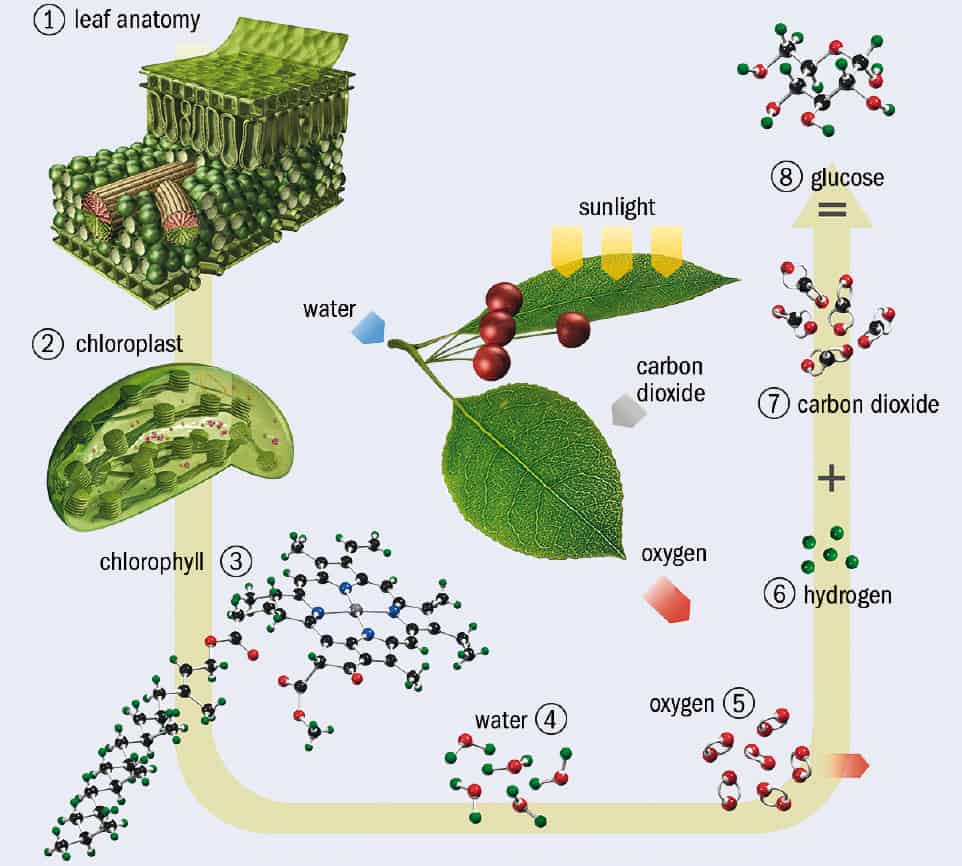
Both plants and photosynthetic bacteria capture solar energy in much the same way (figure 1). Photons of sunlight are absorbed by the chlorophyll molecule and other light-absorbing “chromophores” in a large assembly of proteins and other molecular structures, which together make up a “photosystem” embedded in the so-called thylakoid cell membrane. This energy creates an electronic excited state of the chromophores, and the energy must be channelled towards other molecules in the “photosynthetic reaction centre” – an enzyme that is the site of the light reactions of photosynthesis. Containing the pigments such as chlorophyll, it is surrounded by light-harvesting complexes that enhance the absorption of photons. There, the energy separates positive and negative ions by some significant distance, rendering them unable to neutralize one another by charge recombination. This separation of charge sets up an electrochemical potential that is ultimately tapped to drive enzyme-assisted reactions for making the energy-storage molecule adenosine triphosphate (ATP) – the main reservoir of chemical energy for powering biochemical metabolic reactions in cells (figure 2).

But this transfer of solar energy to the reaction centre is extremely efficient: almost every photon of the captured solar energy is used by the cell. The mystery is how photosynthetic organisms achieve such efficiency in a warm, wet, disorderly environment.
Random hops or straight walks?
Until quantum coherence – the synchronous phase relation between different states of a quantum system – entered the picture, energy transport in photosynthesis was thought to involve interactions between the electronic states of chromophores. The idea was that the energy is passed on to a neighbouring chromophore with a slightly lower energy level. But there was thought to be no coherence – no in-phase resonance – in this coupling. Instead, the energy transport was pictured as a random (incoherent) hopping process between chromophores, guided by an overall energy gradient, like a drunken sailor staggering downhill.
But even as far back as the 1930s it had been suggested that the efficiency of photosynthetic energy transfer might come from some kind of synchronization of the wavelike electronic excitations – known as excitons – between chromophores. In 2007 physical chemist Graham Fleming of the University of California at Berkeley and his co-workers claimed to find evidence that this is indeed what happens. If there really was coherence among excitons, they reasoned, it should be possible to see interference between the different pathways: so-called quantum beats, like the acoustic beats audible when sound waves of similar frequency interfere. Such coherence would allow the excitation to find the optimal route to the reaction centre, without wasting energy through random hopping.
Fleming, with postdoc Engel and others, used a technique called photon-echo spectroscopy to study the energy-transfer process in the so-called Fenna–Matthews–Olson (FMO) pigment–protein complex – the part of the photosynthetic apparatus that mediates energy transfer in the thermophilic bacterium Chlorobium tepidum. This method involved exciting coherent excitons using an ultrashort “pump” pulse from a laser, and then probing the way the excitation evolved over time using subsequent ultrashort laser pulses to look for signs of beating.
At the same time Fleming and his collaborators Hohjai Lee and Yuan-Chung Cheng, also at Berkeley, studied energy transfer between two types of pigments in the photosynthetic reaction centre of a different kind of photosynthesizing bacterium. Although the researchers saw the beats that they had anticipated in both cases (suggesting that coherence was involved) the studies were conducted at temperatures much colder than the physiological conditions under which such organisms operate in nature.
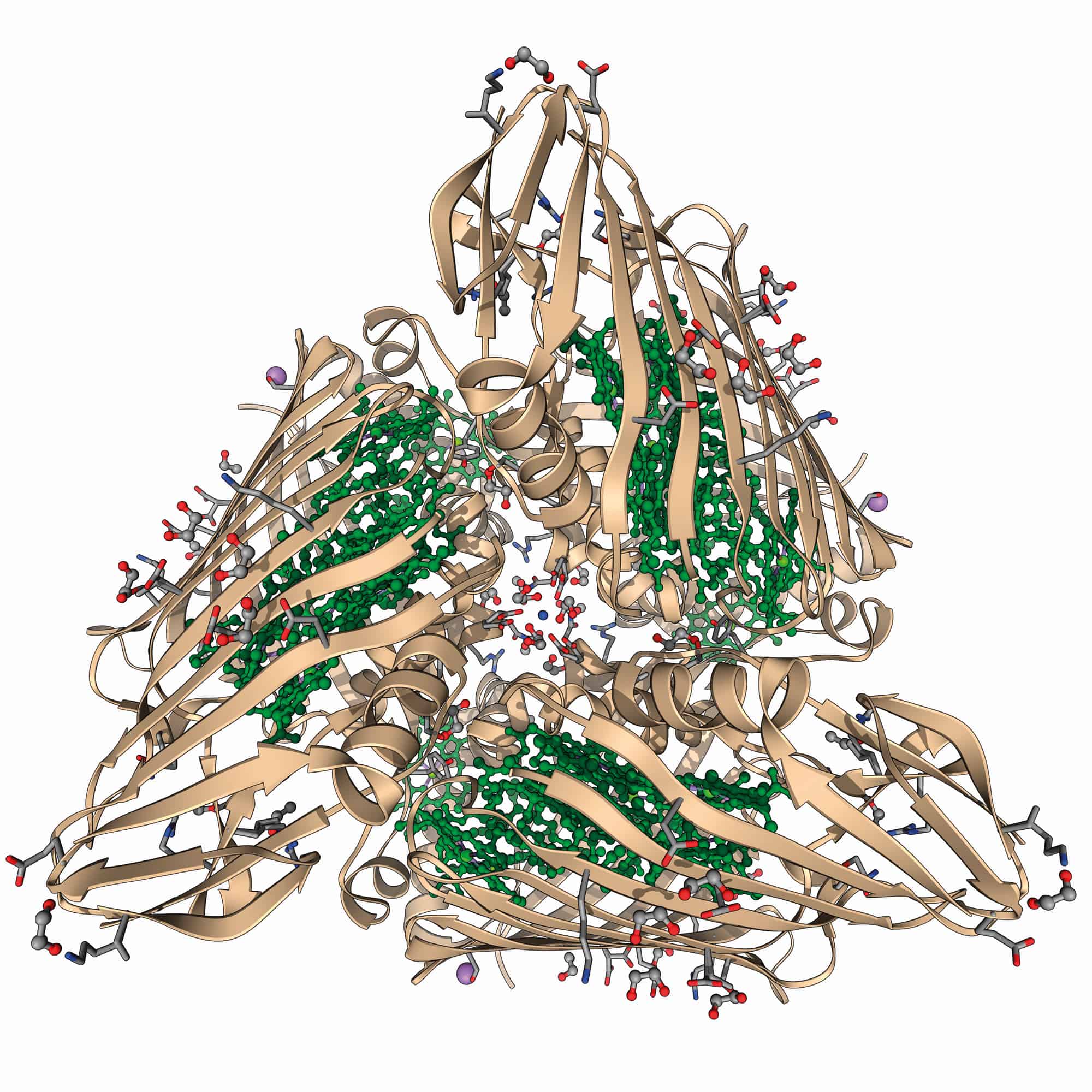
But in 2010 Engel, now in Chicago, reported that quantum coherence in the C. tepidum FMO complex survived even at room temperature. Meanwhile, another of Fleming’s former postdocs, photochemist Gregory Scholes at the University of Toronto in Canada, collaborated with chemist Elisabetta Collini and co-workers to look for such effects under ambient conditions in yet another type of organism: photosynthetic cryptophyte algae, which can harvest light efficiently enough to carry out photosynthesis even in dim conditions. They too saw beats lasting for longer than a tenth of a picosecond at room temperature. This putative electronic coherence, they said, was an order of magnitude longer-lived than had been previously thought – and long enough for the energy to get transferred from the chromophores to the photosynthetic reaction centre.
Natural computing?
This seemed remarkable. Quantum coherence between quantum bits (qubits) placed in entangled states is what physicists are seeking to exploit in quantum computers. But qubits are typically cooled to within a fraction of a degree of absolute zero, and even then, coherence survives only for brief instants, among just a handful of bits, before decoherence sets in due to disturbance from the surrounding environment. So intuition seemed to insist that a quantum effect like this would never survive the warm, wet conditions of living cells. How could photosynthetic organisms sustain it?
But the closer researchers have looked, the more complications and nuances have arisen. Did the early work really detect electronic quantum beating at all? What was actually measured was an oscillating spectroscopic signal; the question is how to interpret it. In 2013 David Jonas of the University of Colorado at Boulder and co-workers argued that the “beats” were in fact caused purely by molecular vibrations – the process of Raman scattering – and not by quantum coherence among excited electronic states (PNAS 110 1203).
A flower’s nano-powers
Engel acknowledges that it was impossible in the original experiments to distinguish definitively between the two possibilities. One way to tell them apart is to make synthetic light-absorbing chemical systems tuned to avoid any coincidence in the energies of excitonic and molecular vibrational energy states – then those two types of state are less prone to coupling. Engel and his co-workers did just that, making dimers of two different molecules that mimicked the light-absorbing groups of photosystems rigidly linked together (Science 340 1431). In 2013 they reported that this system also shows quantum beats that did not appear in the vibrational Raman signals.
But when the same idea was tested by Dwayne Miller of the Max Planck Institute for the Structure and Dynamics of Matter in Hamburg, Germany, and his team using dye molecules joined together in pairs by a more flexible linker, they came to a different conclusion (Nature Chem. 6 196). They argued that the previously observed coherence was too small in amplitude to originate in excitons, and was indeed a kind of classical resonance effect involving molecular vibrations. Besides, they said, any electronic coherence decays too rapidly to have any meaningful biological consequences.
Miller has also now re-examined the behaviour of the FMO complex itself (PNAS 114 8493). “We went back and redid the original work at room temperature and actual physiological conditions, and it is absolute certain there are no long-lived electronic coherences that direct energy transfer”, he says. “The beats are comparable in amplitude, frequency and decay rate to trivial Raman vibrations of the electronic ground state excited in the process. It is Raman that they saw, not long-lived electronic coherence.”
Mixed vibes
Quantum theorist Michael Thorwart of the University of Hamburg agrees. In 2011 he reported calculations of the electronic and vibrational states in the FMO protein which, he says, implied that long-lived coherence wasn’t feasible (Phys. Rev. E 84 041926). “At that time, these theoretical calculations were the best ones available”, says Thorwart. He collaborated with Miller to interpret the recent experimental measurements of excitations of FMO, and he says that the findings match those earlier predictions. Any coherence is tiny and comes from vibrational coupling of the electronic states, somewhat akin to the famous synchronization of wall clocks observed by Christiaan Huygens in the 17th century due to vibrational coupling through the wooden board on which they were mounted.
Increasingly the consensus seems to be that molecular vibrations lie at the core of the disputed observations. And yet Elisabet Romero of the Free University of Amsterdam and her co-workers have carried out spectroscopic experiments that they interpret as evidence that vibrations in the reaction centre of the photosystem from spinach support quantum coherence between excitonic states, by strengthening the interactions between them, and promoting coherent energy transfer (Nature Phys. 10 676).
Very recently, Scholes (now at Princeton University) and co-workers, including veteran photosynthesis expert Robert Blankenship of Washington University in St Louis, Missouri, have told a slightly different tale. They conducted pump-probe spectroscopy on tailor-made mutant versions of the FMO protein complex (Nature Chem. 10.1038/nchem.2910). These variants have different electronic states from the native form, and so if excitons are involved in the spectroscopically observed beats – the characteristic sign of coherence – then these beats should have a shifted frequency. But they don’t: the signal stays just the same. Scholes says this indicates that the oscillations comes purely from vibrations of the electronic ground state (the lowest-energy state). Miller says that this is consistent with earlier work from several other groups, and that “there now seems to be consensus that the original beats were vibrations, not excitons”.
So, while Romero and co-workers’ findings are similar to what Engel originally claimed, Miller and Scholes’ teams are certain that it’s vibronic coupling that is at play. While the beats are real enough, it’s how to interpret them that’s the problem.
Quantum(ish)
Yet Scholes concedes that his new results do support the original contention that “the molecules in the FMO protein are coupled in a special way and this may aid energy transport by directing it or making it quicker”. According to Romero, this tuning of molecular vibrations to the right frequencies for transferring energy makes the photosystem what she calls a “quantum-designed light trap”. When you look at photosynthetic reaction centres for a range of organisms, she says, “there is only one design that is conserved, which suggests that nature has found a design able to perform efficient charge separation and has maintained it”. In other words, she says, natural selection seems to have favoured this quantum-optimized process.
Until quantum coherence entered the picture, energy transport in photosynthesis was pictured as a random hopping process, guided by an overall energy gradient, like a drunken sailor staggering downhill
But Miller argues that the strength of the vibrational coupling is far too low to enhance energy transport. He sees an imprint of evolved optimality in the very absence of quantum coherence – in the fact that it is very rapidly lost after photo absorption through decoherence. “It turns out that nature has evolved to not beat decoherence but exploit it,” he says. It’s precisely because decoherence causes the dissipation of energy that the energy transfer can find its way gradually downhill along the most energy-efficient path, guided by how electronic properties vary from place to place in the molecular environment. “It’s a bit like laying paving stones down a hillside to direct hikers to stay on the trail and not explore the full landscape,” he says.
Full circle
So then, is photosynthesis “quantum” or not? “The observations show that there is correlation between the wavefunctions of the states involved in energy or electron transfer,” says Romero. “But these effects are not considered by some scientists as truly quantum coherence in the sense that entangled states of quantum computing are understood.” And Engel agrees that to compare the two is to invoke “the wrong language”.
Maybe so – or maybe these correlations and coherences, mediated by vibrations, are in any case too weak to have any biological relevance, and we still have to think of exciton states in the photosystem as being more or less localized to particular molecular groups, with incoherent transfer of energy between them. That’s what Miller and Thorwart think. “We have come full circle,” Miller says, “and it seems that the early picture of energy transport as a largely incoherent process has withstood the challenge.”
Romero says that arguing whether energy transport is a true quantum effect or not isn’t terribly productive anyway. “In my view, the relevant issue is to understand how plants or other photosynthetic organisms are able to transfer energy and electrons on an ultrafast timescale in the right direction with high quantum efficiency,” she says. If we understand that, we might be able to do something useful with it. “This knowledge holds crucial lessons about how to engineer human-made systems with the capacity to convert sunlight energy to electrochemical energy to produce electricity or, even better, to produce solar fuels catalytically,” Romero asserts. That’s an idea everyone can get behind – but first we need to know how nature does it.





