Look closely at a cup of coffee or tea and you might see a white mist hovering over the surface. This is thought to be micron-sized droplets of liquid, which physicists know can levitate over the surface of a hot liquid. Sometimes, the levitating droplets can even arrange themselves in a regular 2D array as they hang in the air.
This phenomenon is poorly understood, yet it has important implications for the thermodynamics of evaporation – and could also have a range of applications from chemical manufacturing to delivering drugs to patients.
Newer model
Now, Oleg Kabov and colleagues at Novosibirsk State University and the National Tomsk Polytechnic Research University in Russia as well as Southern Methodist University in the US have seen a similar array of tiny droplets over a hot solid surface. They developed a new model to explain the effect, which they say could also explain the behaviour of droplets over hot liquids.
Droplet levitation over hot dry surfaces is called the Leidenfrost effect and most previous studies have been done with surfaces well above the liquid’s boiling point. However, the team did its experiments on a copper block heated to just 85 °C. This allows the surface to be partially covered by a thin layer of water, allowing them to study levitation over both wet and dry surfaces. The block is monitored by a microscope connected to a high-speed camera that views an area measuring about 1 mm across.
Dry patch
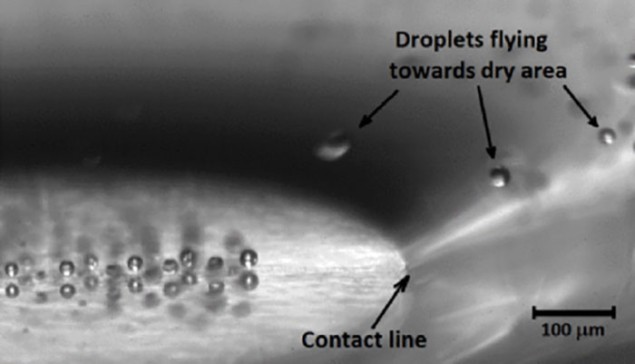
The experiment begins with the copper covered in a uniform layer of water 400 μm deep. A jet of air is fired at the surface, creating a dry patch about 750 μm across. The heated surface is then switched on and droplets begin to form above the liquid layer. Some of these droplets then migrate to the dry patch, where they levitate.
Writing in Physical Review Letters, the team points out that the temperature of the surface is much lower than the conventional “Leidenfrost temperature”, above which a droplet will create an insulating vapour layer that both stops it from evaporating and stops it from falling onto the hot surface.
Vapour reflection
The team therefore had to develop a new model to explain this levitation over a lower-temperature dry surface. Their theory involves vapour flowing out from a droplet and reflecting from the copper surface, thereby causing the levitation. They also reckon that this outflow of vapour creates a repulsive interaction between droplets, which causes multiple droplets to form regular arrays.
Once they developed their model for dry surfaces, Kabov and colleagues revisited droplets over wet surfaces and concluded that the same evaporative effect is responsible for levitating droplets, and the formation of regular arrays.