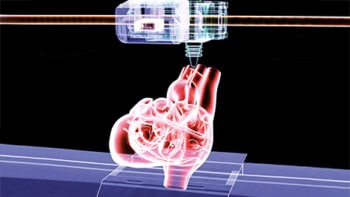
The liver is one the most important organs for when it comes to assessing drug toxicity, but animal models are often very bad at predicting how humans will react to drugs or to toxic biothreat agents. Researchers at Harvard University recently succeeded in bioprinting a new liver-on-a-chip platform that offers a promising solution, as they reported recently in the journal Biofabrication. We spoke to Su Ryon Shin of Harvard Medical School about her group’s new bioreactor.
Could you tell us a little about your work and your new platform?
The liver plays a critical role in metabolizing drugs and detoxifying blood. Drug-induced hepatoxicity is one of the main reasons that drugs do not make it past clinical trials so researchers need to develop good in vitro models for evaluating hepatoxicity. Our perfusable bioreactor is an important step forward towards such a model and has proved to be excellent for evaluating drug toxicity. It can even be extended to make multiple organ-on-a-chip systems by connecting a second or even third bioreactor.
Bioprinting is an easy way to make precisely controlled 3D architectures that can be combined with miniaturized bioreactors to make next-generation organ-on-a-chip platforms. Such bioreactors contain tissue that has been engineered under continuous perfusion so that the cells being cultured receive a constant supply of nutrients and oxygen. It is easy to access tissue constructs within the structure and assess how they behave at different times during culture.
Among the various 3D cell culture models, multicellular spheroids, formed by cell aggregates, show promise. For one, the oxygen tension in the core of the spheroid is very different to that in the periphery, a situation that mimics the real-life in vivo oxygen gradient in hepatic lobules. Studying such cell spheroids instead of dispersed cells therefore allows us to better evaluate how cells respond to different drugs.
Another advantage of these spheroids is that we can encapsulate them with hydrogels that are compatible with rapid fabrication techniques, including bioprinting, which allows us to make complex architectures similar to those found in vivo. And this is exactly what we did in our platform for culturing 3D human HepG2/C3A spheroid cells.
How does your work fit in with your wider research programme?
My research team is actively involved in studying minimally invasive approaches to testing drug toxicity in general. These studies involve biomaterials research, tissue engineering, microfluidics, microfabrication, organs-on-chips, biosensors and bioprinting. Developing organs-on-chips is one of our central research areas for developing in vitro testing platforms.

Kidney chip targets more efficient drug development
Since the publication of our Biofabrication paper, my colleagues and I have also been working on developing a body-on-a-chip platform, which is a modular, scalable microfluidic system with multi-organ system integration (mimicking human organ function). We achieved this by creating resealable microfluidic devices capable of cell culture and analysis.
We have also recently developed multisensory-integrated organs-on-chips platforms for automated and continual in situ monitoring of organoid behaviour. This work was published in PNAS. Using the same technique, we are now developing bio-mimicked human organoids using patient-derived cells to further improve these systems.
How has the research community reacted to your work?
We are working with interdisciplinary scientists (clinicians and engineers, for example) from around the world and have received more than 12 National Institutes of Health (NIH) and Department of Defense (DOD) grants so far.
Especially relevant to the publication in Biofabrication is a NIH-U01 grant with Shiladity Sengupta, assistant professor at Harvard Medical School, who is developing novel therapeutic strategies for regenerative medicine.
We have also received an R01 grant with Jingyong Ye, associate professor at the University of Texas San Antonio to develop highly sensitive biosensors for real-time monitoring of cardiac organs-on-a-chip. Among our other projects is a collaboration with clinicians at the Mayo Clinic to build brain tumours-on-chips.
How is your research progressing, and what are your future plans?
Since our Biofabrication paper, we have been developing smart bioreactors in which we can control the amount of oxygen and the applied electrical signal. We have succeeded in building various types of organoids, including heart and liver, as well as breast cancer and brain tumour tissue using the bioprinting technique. We have also developed an immune-on-a-chip to evaluate nanoparticle toxicity.
Our future work will focus on developing efficient methods for accurately analysing both drug efficacy and toxicity in a personalized organ-on-a-chip system using patient-derived normal or cancer cells. This platform aims to reduce trial and error when treating real-world patients and will also improve clinical predictions of how they respond to different chemotherapeutics.
Further details on the study can be found in the journal Biofabrication.
- This article is one of a series of reports reviewing progress on high-impact research originally published in the IOP Publishing journal Biofabrication.