Artificial scaffolds created by 3D printing of polymeric materials have become a focus for tissue engineering research for applications such as repairing cartilage in patients with osteoarthritis. A team at the University of Saskatchewan in Canada recently investigated how the molecular weight and shapes of pores in scaffolds made from poly(e)-caprolactone (PCL) affect their mechanical properties, which ideally should match the type of cartilage being treated. Adeola Olubamiji, lead author of the study – which was originally published in the journal Biofabrication – talks to Physics World about her group’s findings as well as more recent progress.
Could you give us a little background to your work?
Cartilage tissue engineering involves fabricating 3D constructs that mimic the biological and mechanical properties of human articular cartilage. Although naturally occurring biomaterials such as collagen, hyaluronic acid and chitosan show promise here, especially because they promote chondrogenesis and mimic the biochemical properties of cartilage, they are expensive and are not very strong mechanically. They also degrade fairly quickly. Because of these drawbacks, researchers are looking for alternative, easily-sourced synthetic polymers that have better mechanical and degradation properties.
What sorts of polymers could be employed in tissue engineering?
Synthetic polymers such as polyglycolide (PGA), poly(L-lactide) (PLLA) and poly(d,l-lactic-co-glycolic acid) (PLGA) are routinely employed in scaffold-based tissue engineering applications. The problem here, however, is that these material scaffolds are too rigid. Researchers have recently found that adding PCL to PLLA reduces this polymer’s rigidity while increasing its visco-elasticity and flexibility.
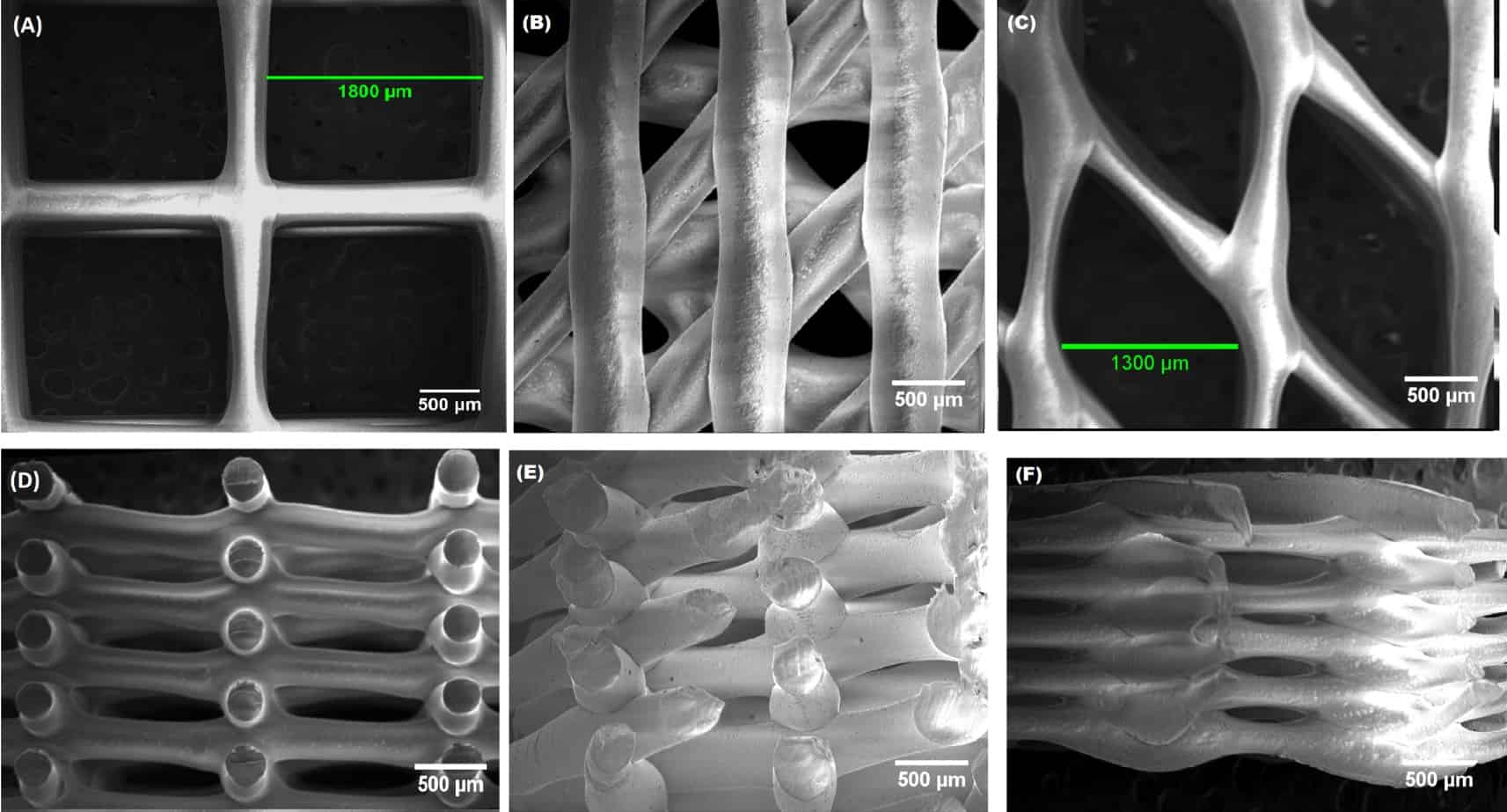
PCL is also good in that it can be reabsorbed into biological tissue, degrades slowly (over time periods of months to years), is cheap, biocompatible and non-immunogenic. In our Biofabrication paper, we looked at how to optimize 3D-printed PCL scaffolds for cartilage tissue-engineering applications.
Could you describe this study and the main results you obtained?
We studied how the molecular weight of PCL and the geometry of the pores in scaffolds made with this material affected its mechanical properties. We also looked at how the polymer strand size, strand spacing and strand orientation affected these properties.

3D bioprinting smoothes path towards cartilage repair
The results show that the molecular weight is important for mechanical properties such as the compressive moduli and yield strength of the 3D-printed PCL scaffolds. Indeed, we found that PCL with a molecular weight of 45 K was a good choice for making viscoelastic, flexible PCL scaffolds that can bear weight – which is an important function of natural cartilage. As for the strand properties, we found that strand size, spacing and orientation all affect the tensile moduli of the scaffolds, but that only strand size and spacing significantly affect the scaffolds’ compressive moduli and porosity.
All in all, our study shows that modulating the PCL’s molecular weight and the shape of its pores can help in the design of PCL scaffolds with mechanical and biomechanical properties that better mimic the mechanical behaviour of human articular cartilage.
How has your work moved on since the publication of your biofabrication paper?
We have successfully implanted 3D-printed hybrid scaffolds in small animal models, with the aim of regenerating articular cartilage damaged by osteoarthritis. We will now be modifying these structures and looking at how they behave in larger animal models.
Biodegradable scaffolds show promise for cartilage repair and we believe that we can make significant advances in these constructs by engineering biodegradable structures with carefully controlled microarchitectures.
- This article is one of a series of reports reviewing progress on high-impact research originally published in the IOP Publishing journal Biofabrication.



