A new motion-correction method for single-molecule localization microscopy (SMLM) lets researchers measure the position of individual molecules with unprecedented accuracy. By stabilizing SMLM images in real time, a team at the University of New South Wales (UNSW) determined the distance between individual proteins on the surface of human immune cells with single-nanometre precision. This improvement in SMLM resolution could lead to greater understanding of cell signalling, but the technique might also be applied to other high-precision instruments such as DNA sequencers and atomic force microscopes.
Observing cellular processes at the level of individual molecules requires images with a resolution that’s impossible to achieve using conventional microscopy: at optical wavelengths, the best resolution permitted by the diffraction limit is far too coarse. In recent years, however, researchers have found a way to get around this limit using a family of techniques known collectively as SMLM.
In SMLM, target molecules are labelled with fluorescent markers that emit for brief periods at random intervals. An image captured at any given moment might record just a few individual excitation events, but by stacking thousands of frames acquired over hours or days, a comprehensive molecular map can be built. As long as the emission events in each frame are spatially separate, the accumulated signals can be statistically fitted to locate the source of each molecule to the nearest nanometre.
That, at least, is how it works under ideal conditions. In reality, minuscule movements of the camera over the course of the process mean that, until now, researchers have struggled to pin down molecules’ positions with a precision better than 20–30 nm.
“There are a number of things that cause drift in the instrument,” says team leader Katharina Gaus. “The biggest cause is probably vibrations – from people walking in adjacent rooms and corridors, for example. All buildings also have a natural frequency, and vibrate when cars (or, in our case, the tram) go past outside.”

Gaus and her colleagues developed a way to compensate for this motion using three separate techniques. First, they placed 3 µm polystyrene beads on the imaging stage (out of the camera’s field-of-view) as fiducial markers. When illuminated with an infrared laser, the beads produced diffraction rings that revealed any relative motion between the sample and the rest of the instrument. These measurements were passed into a feedback loop that corrected the sample’s position 12 times per second, limiting motion to less than 1 nm over a 20 hour period.
The team set up another feedback loop by integrating a white LED into the microscope body and focusing it on the corner of the camera’s sensor – an electron-multiplying charge-coupled device (EMCCD). This produced an optical fiducial whose intensity peak could be located with a precision of 0.05 nm. Any change in this position represented a drift in the fluorescence signal, which the researchers corrected automatically with a piezoelectric mirror.
The third correction addressed potential discrepancies in how the camera’s EMCCD registered the positions of green or red emitters. To detect and compensate for such discrepancies, the researchers measured the sensor’s responses to a nanohole array filled with green and red dye.
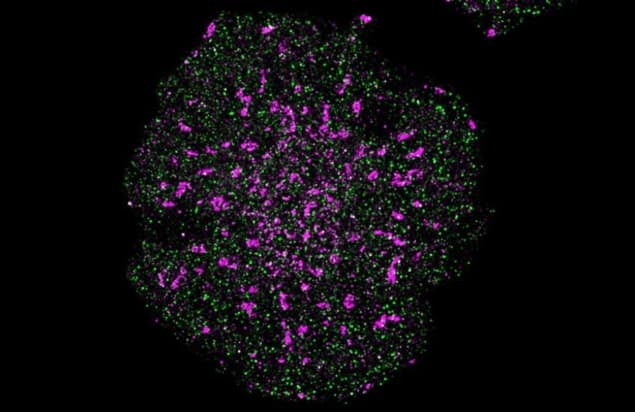
Gaus and colleagues tested their technique – which they call Feedback SMLM – by measuring the positions of signalling proteins on human T cells. They found that the process of T cell activation is determined by the distances between specific proteins, and that a difference in separation of just 4–7 nm distinguishes one cellular response from another.
Although discerning such tiny distances would be impossible using conventional SMLM, it is within the scope of an existing method called fluorescence resonance energy transfer (FRET). In this technique, the varying proximity of two fluorophores is reflected in changes to their emission intensity. However, Gaus thinks that Feedback SMLM could replace FRET, as the latter technique is blind to inter-molecule distances larger than 10 nm, and is also affected by other factors including the fluorophores’ dipole alignment and the pH of the environment.
Full details of the research are reported in Science Advances.