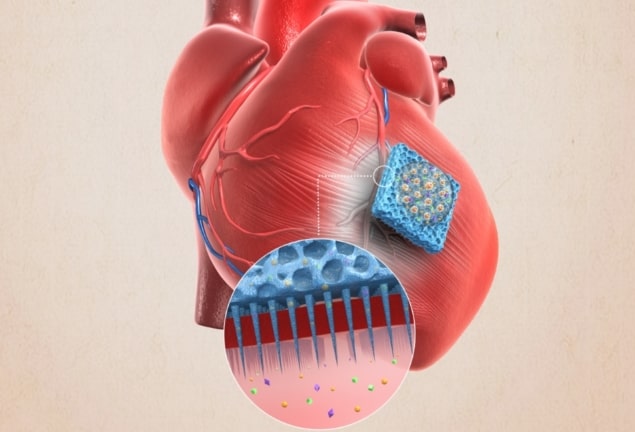
Researches at North Carolina State University (NCSU) and University of California, Los Angeles (UCLA) have developed a biocompatible microneedle patch topped with a layer of cardiac stromal cells (CSCs) enveloped in fibrin gel. When positioned on damaged regions of a rat’s or pig’s heart, the patch led to heart repair and protection of cardiac function (Sci. Adv. 10.1126/sciadv.aat9365).
Myocardial infarction, known as heart attack, happens when a blockage prevents blood flowing to the heart, causing heart cells (cardiomyocytes) to starve and die. The patient’s outlook is bleak: scar tissue will replace the injured heart muscles; genetic, structural and biomolecular changes will then occur at the affected site, a process known as heart remodelling. Survivors typically face higher risks of developing heart failures in the future. There is, therefore, a need for therapies that can reduce the size of heart scar tissue and prevent heart remodelling.
In the past 20 years, stem cell therapy has been in the spotlight due to its ability to promote tissue healing by secreting growth factors (molecules capable of stimulating cell growth). Previous studies and clinical trials showed that directly injecting CSCs (a cardiac stem cell) with syringes can regenerate damaged cardiac tissues. However, this injection method is not ideal because of the high stem cell loss at transplantation sites. Instead, researchers are attempting to create various stem cell delivery systems that can improve cell retention rate on injured heart muscles.
Pushing the boundaries of therapeutic cell delivery
A research team led by Ke Cheng at NCSU and Zhen Gu at UCLA developed a biocompatible patch studded with an array of 5 μm microneedle tips. They then covered the top of the patch with a protein-based, biodegradable fibrin gel that encases the heart stromal cells. They call this CSC-biomaterial hybrid a microneedle patch (MN-CSC). Looking like a mini velcro tape, the tips not only secure the patch’s position on injured heart muscles, but also act as channels capable of releasing growth factors from CSCs.

Next, the research team incubated the MN-CSC with cardiomyocytes derived from neonatal rats. They found no inhibition in cell growth and indeed, the heart muscle cells contracted. In subsequent tests with rats, the researchers induced myocardial infarction, then attached a 0.5 x 0.5 cm MN-CSC patch to the rat’s heart. They found that the implanted patch did not cause an elevated infiltration of T cells (immune system cells that recognize and destroy foreign pathogens or infected cells), demonstrating that the heart tissue did not reject the patch.
In the final part of this study, the authors used pigs as experimental subjects, due to the close resemblance between blood flow mechanisms in pig hearts and human hearts. The results showed the MN-CSC patch was non-toxic to pig heart tissue. The team also reported that the patch improved left ventricular ejection fraction (LVEF) in pigs’ hearts treated with MN-CSC. LVEF measures the percentage of blood leaving the left ventricular each time it contracts and is an excellent indicator of heart function.
Further research is needed to optimize the design of the MN-CSC patch. Will by-products from degradation of the biomaterials affect animals or even the human body? Can researchers devise a minimally invasive method to implant this MN-CSC patch to the heart, as opposed to an open-heart surgery? Without a doubt, the tissue-regenerating, non-toxic device may push the boundaries of heart regeneration.



