Researchers at University College London have developed a minimally invasive cancer therapy that uses magnetic resonance navigation (MRN) to steer a ferromagnetic thermoseed through tissue to a target tumour. Once in place, the thermoseed is remotely heated to a high temperature, destroying the cancer cells via thermoablation. The technique, called MINIMA (minimally invasive image-guided ablation), could provide an alternative to surgery or radiotherapy for treating brain and prostate cancers.
MRN works by using the magnetic field gradients generated by the imaging gradient coil of an MRI scanner to steer the thermoseed within the body. The scanner can also image the seed in real-time, enabling its accurate navigation to target locations. The high precision of this emerging treatment could help reduce damage to adjacent tissue, limiting debilitating side effects.
Writing in Advanced Science, the researchers explain that MINIMA has the potential to combine diagnosis and therapy into a single MRI theranostic device that enables both tumour localization and treatment on the same platform.
“MR images will help determine the least invasive path along which the thermoseed will be navigated, and using imaging, the position of the thermoseed can be constantly assessed, giving real-time assurance of the thermoseed’s location,” explains senior author Mark Lythgoe. “Once at the target, an alternating magnetic field may be applied, causing the thermoseed to heat and deliver localized cell death. The thermoseed may then be navigated through the target tissue, heating at multiple locations until the whole region has been ablated. After the thermoseed is navigated back to the point of entry into the body and removed, MR imaging could assess the success of the procedure.”
Proof-of-concept study
In a preclinical study, Lythgoe and colleagues demonstrated precise thermoseed imaging, seed navigation through ex vivo brain tissue tracked to within 0.3 mm accuracy, and successful eradication of tumours in a mouse model. They also investigated the feasibility of using an MRI scanner with the capability to turn the magnetic field off and on, to ensure safe insertion and extraction of the seed.
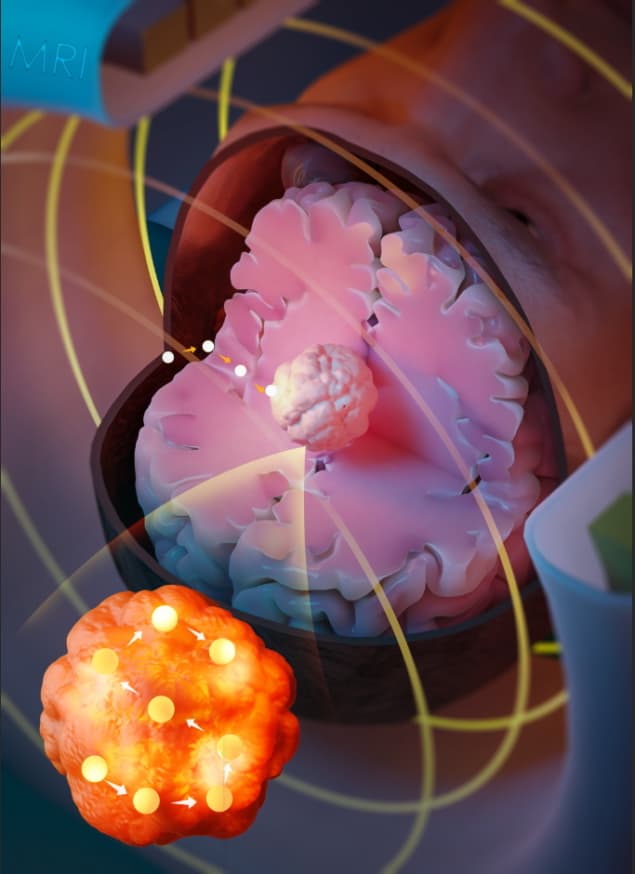
The team used a 2 mm-diameter chrome steel sphere as the thermoseed, selected because chrome steel has a high saturation magnetization, which increases the generated translational forces and improves tissue penetration. The seed’s spherical shape enables ease of movement in all directions, while its size is comparable to standard brain biopsy needles.
The researchers performed navigation experiments using a 9.4 T preclinical MRI system and a clinical 3 T system. They employed a frequency-selective MRI method that exploits image distortion artefacts around the thermoseed to pinpoint its location. To heat the seed, they used a custom-built MR-compatible magnetic alternating current hyperthermia system to generate an alternating magnetic field of up to 8 kA/m at 700 kHz.
Thermoseed movement was initiated in steps, with imaging after each step to confirm that the seed was steered precisely along the preplanned path. This repeat imaging enabled a high level of control over seed movement, by adjusting the applied force based on continuous assessment of the movement. The distance moved by the seed increased as the gradient strength increased, although certain tissue structures could hinder motion. Increasing the seed diameter and the duty cycle also improved tissue penetration and the efficiency of movement.
In studies in mice, a 5 min thermoablation successfully destroyed the tumour bulk, with new growth undetectable in a group of animals followed for up to 33 days. Cell culture research showed that the ablation volume could be controlled by varying the heating duration.
“One of the major advantages of MINIMA is that it may be applied to different tissues and diseases,” notes first author Rebecca Baker. “For example, the thermoseed could be guided through the brain to ablate an area causing seizures in patients with drug resistant epilepsy, possibly limiting damage to healthy tissue.”
Magnetomechanical stimulation
While MINIMA employs the imaging gradients inside the MRI bore to steer the thermoseed for precise tumour ablation, the team has developed another novel technology that utilizes the static fringe magnetic field at the edge of the bore to selectively stimulate astrocytes, star-shaped glial cells found in the brain and spinal cord.
“Astrocytes are implicated in a range of brain disorders such as epilepsy, stroke and depression,” explains co-author Yichao Yu. “They are also highly sensitive to mechanical stimuli.”

Taking advantage of this property, the team developed a technique called magnetomechanical stimulation (MMS). Here, magnetic particles smaller than 1 µm are targeted to the cell membrane of astrocytes and, upon application of a magnetic field, generate a force that stretches the cell membrane. This activates the release of adenosine triphosphate (ATP), a signalling molecule that can influence the activity of neighbouring cells.
This ability to remotely stimulate astrocytes offers potential to treat certain brain and mood disorders, including severe depression. Indeed, animal models have shown that ATP released by astrocytes in certain brain regions has a potent anti-depressant effect.
“Because astrocytes are sensitive to touch, decorating them with magnetic particles means you can give these cells a tiny prod from outside the body using a magnet, and as such, control their activity,” says Lythgoe. “We are very excited about this technology because of its prospect as a neuromodulation therapy.”
Like MINIMA, the technique achieves both imaging and actuation using a single MRI system. The team demonstrated that they could assess the delivery of the particles to a specific brain region using an MRI scan and then induce MMS with the fringe field of the scanner.
Three-prong photothermal therapy eliminates tumours in mice
“Because MMS exploits the intrinsic mechanosensitivity of astrocytes, it does not require genetic modification of the target cells as existing cell control technologies such as optogenetics and chemogenetics do. This removes a major obstacle to clinical translation,” comments Yu. “Moreover, the common existence of MRI scanners in hospitals mean that it may be possible to implement MMS without any additional hardware.”
In the future, the researchers plan to make MMS minimally invasive. Currently, particles are injected directly into the brain via a surgical opening in the skull. Instead, they hope to use methods such as MR-guided focused ultrasound to disrupt the blood–brain barrier, allowing particles injected into an arm vein to travel to a specific brain region via the leaky brain barrier.




