Finding ways to effectively utilise the vast quantities of sunlight that impart energy to our planet remains the greatest challenge for many alternate energy visionaries. Among them is Wee-Jun Ong who is at the forefront of research into photocatalysis. As part of a publication showcasing promising new methods for solar energy conversion, Ong has stated that “accomplishing solar-to-energy conversion will, in turn, lead to the development of a sustainable, green, and renewable future without environmental detriment. All of us need to do our roles to make this world better.”
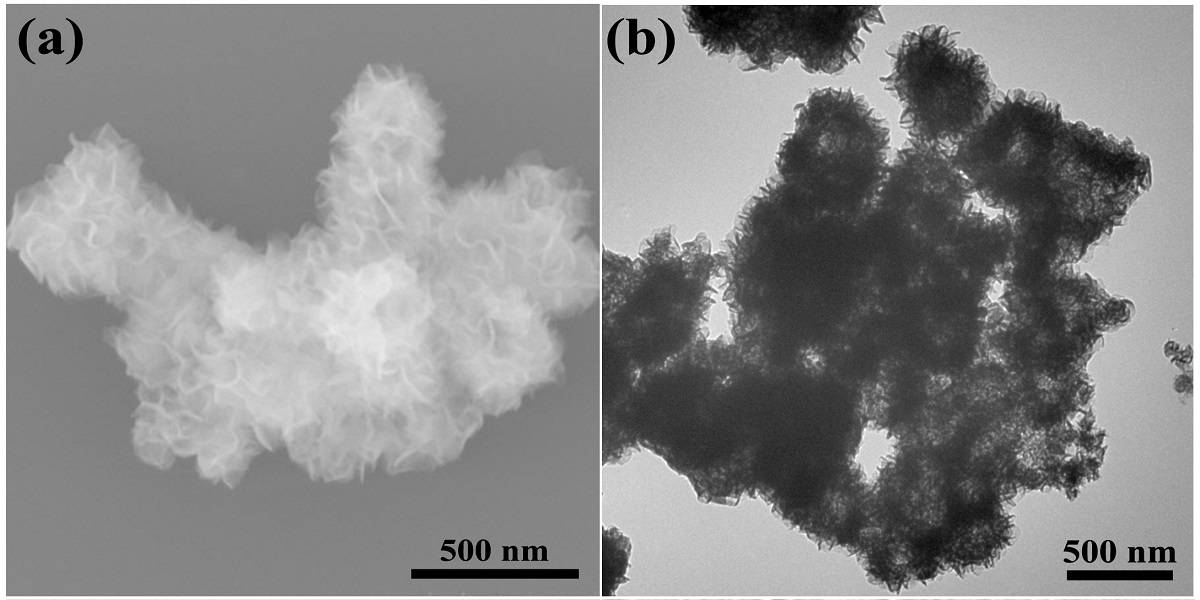
Reporting in ChemSusChem Ong and colleagues show that submicron flower-like structures created by a one-pot production method have high photocatalytic activity, specifically for water splitting to produce hydrogen. The work, a collaboration between Singaporean and Chinese institutions, represents the forefront of visible light-driven hydrogen evolution research that is both effective and environmental. The nanostructures do not contain precious metals and so could provide a path toward cost-effective and environmentally friendly energy conversion.
In combining both sunlight and water to create hydrogen, lead author Deqian Zeng and colleagues including Ong make use of naturally abundant resources, a factor that is key in providing a sustainable future for us all. This hydrogen can be used as a clean fuel source, but arguably more important is the alluring potential that has been unearthed in using hierarchical nanostructures for potential application to a wide variety of energy storage and conversion applications.
Less precious, more efficient
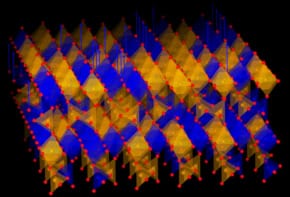
Zeng et al. present an attractive alternative to current water-splitting processes, which rely heavily on precious metals. The use of precious, or noble, metals is not ideal as the metals are just that: precious as they are in limited supply, and therefore expensive both in terms of monetary cost and the environmental impact in mining them. By combining ZnIn2S4 and MoSe2 heterostructures, the researchers have developed novel photocatalysts from abundant materials with highly desirable properties including non-toxicity, high chemical stability and superb catalytic activity.
The properties are particularly impressive considering that the production method is much quicker and much simpler than competing processes: a reaction duration of just one hour makes use of a solution-phase hybridization approach that needs only a single pot to fabricate. This is a marked improvement compared with alternate methods that traditionally involve complex, multi-step reactions over the course of days.
Nanoflowers for photocatalysis

To elucidate the role of nanostructures in producing a highly efficient photocatalyst, a range of state-of-the-art techniques such as X-ray diffraction, electron microscopy and energy-dispersive X-ray (EDX) mapping were employed. EDX is an incredibly useful spectroscopic tool that enables elemental analysis down to the nanometre length scale, and in this instance it shows the coexistence of Zn, In, S, Mo and Se in the hybrid system (see image attached to article). Further morphological insights from the remaining characterisation techniques delve into how the structure gives rise to high chemical reactivity.
Ong explains that “importantly, the MoSe2 nanonetworks have multiple pores and are in favour of the direct light absorption of ZnIn2S4, even though the ZnIn2S4 is hybridised with MoSe2. The ZnIn2S4/2%MoSe2 photocatalyst displays a dramatically high noble-metal-free hydrogen generation rate of 2228.
This system signifies a massive enhancement in the production of hydrogen, weighing in at over two times larger than similar systems. The apparent quantum yield is a measure of how many reactive electrons are produced depending on the amount of incident light (at a 420 nm wavelength in this instance, which corresponds to violet light); in the world of photocatalysis, 21% is a very impressive figure.
To the future and beyond
To fully investigate the mechanisms and applications of this material, various photoelectrochemical tests were carried out, with promising performances demonstrating that this system can be used as a basis for future commercialisation. However, the work does not stop there, and as Ong reveals to Physics World, “besides water splitting, my research direction is now gearing toward diverse types of energy conversion, including photocatalytic, photothermal, photoelectrochemical and electrochemical CO2 reduction and N2 fixation.” So watch this space for even more future energy solutions.
Full details of the research are reported in ChemSusChem, published as an invited contribution to a Special Issue of the journal ChemSusChem, Artificial Photosynthesis for Sustainable Fuels by the Editor-In-Chief. DOI: 10.1002/cssc.201701345