Radiotherapy plays an essential role in the management of cancer, but unwanted irradiation of healthy tissues can lead to adverse side effects and limit the deliverable tumour dose. Radiosensitizers, which preferentially sensitize tumour cells to irradiation, can increase the therapeutic window and enable higher target doses without increasing normal tissue damage.
One approach under investigation to improve the effectiveness of radiotherapy is the use of nanoparticles as radiosensitizers. And at the recent ESTRO 2020 congress, attendees heard about some of the latest developments and clinical studies in this field.
Dual-purpose particles
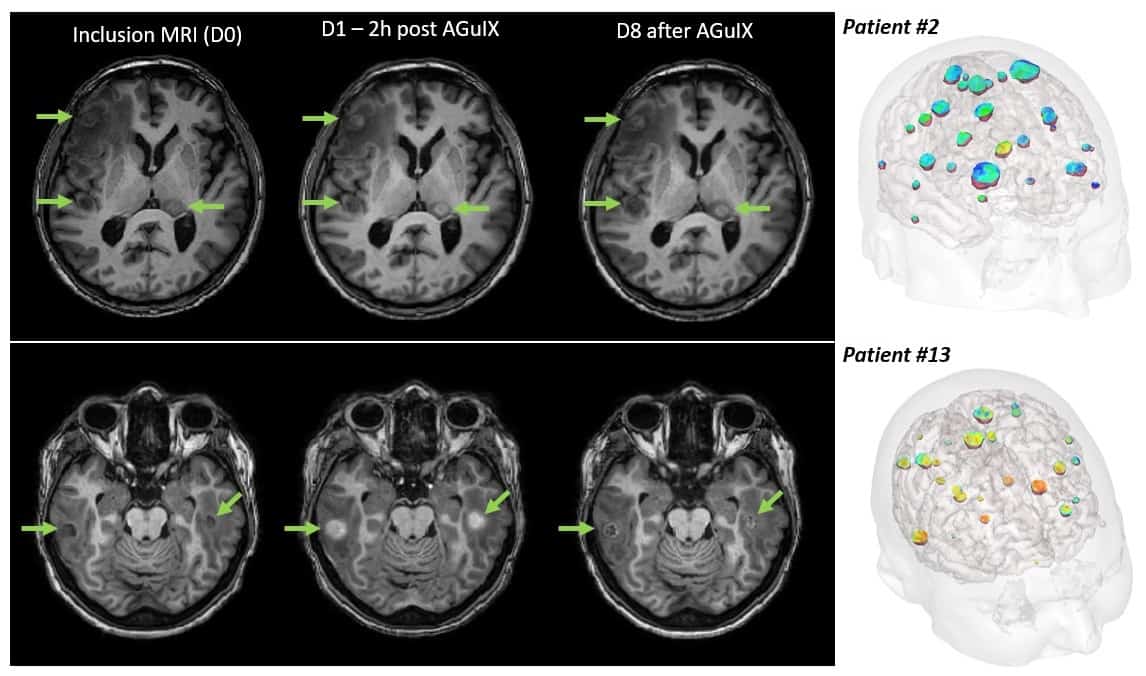
At an ESTRO session focused on novel technologies, Camille Verry from Grenoble Alpes University Hospital described the first-in-man study of gadolinium-based AGuIX nanoparticles as radiosensitizing agents. AGuIX nanoparticles, which are around 4 nm in size, are also MRI contrast agents, enabling visualization of their localization.

In a phase I study, NANO-RAD1, the researchers treated 15 patients with multiple brain metastases from melanoma, or lung, breast or colon cancer. After intravenous injection with the nanoparticles, the patients received a 30 Gy dose of whole-brain radiotherapy in 10 fractions. The team used five AGuIX dose levels, from 15 to 100 mg/kg, to determine the maximum tolerated dose.
“The study design is simple, we give one injection on the day of the first radiotherapy fraction,” explained Verry. “Two hours after the injection, we perform MRI for each patient to see the nanoparticle distribution, and then perform the radiotherapy.”
MR images showed that AGuIX localized in all of the metastases, regardless of primary tumour type, with no nanoparticles seen in healthy brain tissues two hours after injection. The results also revealed that the nanoparticles were safe at all dose levels. “There was good immediate tolerance, no pain, no systemic reactions and no local complications,” said Verry. “So we can treat with 100 mg/kg, which is now the recommended phase II dose.”
The team also noted that at seven days post-injection, AGuIX was still present in all the brain metastases, albeit at a lower level. “That’s good news if you want to perform dose enhancements with daily radiotherapy,” added Verry.
Of the 14 evaluable patients, 12 experienced a clinical benefit from the treatment, with a decrease in tumour volume. The overall survival was approximately 5.5 months and roughly one third of patients were alive at 12 months.
The researchers also analysed each metastatic lesion (255 in total) before and 28 days after treatment. They observed a correlation between AGuIX uptake and tumour response, with increased AGuIX in the tumour leading to a greater clinical response one month after treatment.
Verry described two example case studies. In the first, a male with lung cancer and eight metastases that had not responded to chemotherapy, radiotherapy with AGuIX at 15 mg/kg reduced the tumour volume from 45 to 15 cm3 three months after treatment. In a second example, a woman with pulmonary adenocarcinoma and 28 metastases was treated with whole-brain radiotherapy plus AGuIX at 100 mg/kg. This led to a large reduction in the number of metastases at up to nine months after treatment.
“We have performed the first administration in humans of AGuIX,” Verry concluded. “The immediate tolerance of this treatment is good and the nanoparticle distribution is favourable, with very important tumour uptake without nanoparticles in healthy brain.”
The next steps are to demonstrate the efficacy of this approach, optimize AGuIX administration and learn about any long-term toxicities. To achieve this, the team had launched a phase II clinical trial, which is currently recruiting in 15 French hospitals. The trial will include 100 patients with multiple metastases not suitable for stereotactic radiotherapy or surgery, randomized to receive whole-brain radiotherapy with or without AGuIX nanoparticles.
Dose enhancement
Speaking in the same ESTRO session, Christophe Le Tourneau from Institut Curie described the use of radiotherapy-activated hafnium oxide nanoparticles for treatment of head-and-neck squamous cell carcinoma (HSNCC). The standard-of-case for patients with unresectable locally advanced HNSCC is concurrent chemoradiation. But chemotherapies do not confer much benefit in older patients and their prognosis is poor, with a median overall survival of 12-13 months.
To address this shortfall, Le Tourneau and collaborators aim to use the non-toxic hafnium oxide nanoparticle NBTXR3 to boost the effects of intensity-modulated radiotherapy (IMRT).

“NBTXR3 is designed to trigger cellular destruction, first by multiplying the number of electrons that are produced when they are irradiated, and also to prime the immune response,” said Le Tourneau.
With these nanoparticles present, he explained, the dose delivered during radiotherapy is multiplied by nine times at the cellular level compared with irradiation alone.
The team’s Phase I dose escalation study (performed in five centres in France and Spain) included 19 elderly patients with stage III or IVa HNSCC who were older than 65 and ineligible for chemotherapy. The investigators tested four nanoparticle dose levels, calculated as 5, 10, 15 and 22% of the primary tumour volume.
Patients received a single intra-tumoural injection of NBTXR3, followed by 35 2-Gy radiotherapy fractions over seven weeks. The study participants experienced no dose-limiting toxicities or serious adverse events (SAEs) related to the nanoparticles. As such, the team defined the recommended dose as 22% of baseline tumour volume. “Interestingly, nine out of 13 evaluable patients treated at a dose of 10% of volume or more had a complete response of the treated tumours,” said Le Tourneau.

Bimetallic nanoparticles enhance proton dose
Next, the researchers performed a dose expansion study including 44 patients from 12 centres across Europe. Patients had a median age of 70 and many comorbidities. In this group, 7% of patients had at least one SAE due to injection procedure or the nanoparticles. There were also 21 SAEs related to radiotherapy, but these toxicities were as expected with IMRT alone. Overall, 67.7% of the patients had complete response of their primary tumour.
“The intra-tumoural administration of NBTXR3 prior to IMRT is well tolerated and safe in frail HNSCC patients with multiple morbidities,” Le Tourneau concluded. “We saw an objective response in 83.9% of patients who were evaluable. Recruitment into the dose expansion trials is now being finalized. And based on these promising results, a global randomized phase III trial is being planned.”