In autoimmune disorders, such as multiple sclerosis (MS) or type I diabetes, the immune cells that normally protect the body mistakenly attack healthy cells. In MS patients, this immune attack damages the myelin sheath that shields nerve cells in the brain and spinal cord, and in those with type I diabetes, the insulin-producing islets of Langerhans in the pancreas are attacked and eventually destroyed.
Stem cells are known to respond to damaged tissue and modulate the immune response, but trials using stem cells to treat autoimmune disorders have had mixed outcomes. Mesenchymal stem cells get trapped in the filter of the lung vasculature bed, with only 1% reaching damaged tissue. Despite this entrapment, there are some therapeutic effects, which led scientists to believe that these stem cells must release something that acts at the inflamed tissue. Researchers have begun to examine the effect of exosomes – nano-sized bundles of molecular components released from cells.
Exosomes have been examined in cancer and type I diabetes, and now for the first time, researchers from the University of California, Irvine, have shown a neuroprotective capability of stem-cell derived exosomes, and identified players in the exosome mechanism of immune-regulation (ACS Nano 10.1021/acsnano.9b01004).
“We showed that due to their size, these vesicles do not get trapped in the lung, and they also target the damaged area and end up into the spinal cord,” says Reza Mohammadi, one of the study’s lead authors.
Interfering with MS
Mohammadi and colleagues used serial ultracentrifugation steps to isolate the (approximately) 115 nm-diameter exosomes from human mesenchymal stem cells the experimental autoimmune encephalomyelitis (EAE) mouse model of MS.
Clinical scoring of the disease revealed improved motor skills and a sustained recovery in exosome-injected mice. And upon dissection, a reduction in de-myelination and regeneration of the protective neural sheaths was discovered.
More potent still was treatment with exosomes released after interferon-gamma stimulation of stem cells. The inflammatory cytokine triggers stem cells and the derived exosomes were shown here to promote neuroprotection in the spinal cord.
Seeking to understand how the exosomes mediated such a neuroprotective response, the scientists delved into the molecular response within the spinal cord. Using a special regulatory T cell reporter model of EAE, a reduction in pro-inflammatory immune cells and a corresponding increase in regulatory immune cells were noted in the spinal cord. In vitro studies confirmed the ability of exosomes to induce regulatory T cells in mouse splenocytes.
Molecular players a plenty
Digging further into the exosome mechanism, Mohammadi performed deep RNA sequencing of exosomes, and identified a plethora of RNA coding for the production of anti-inflammatory proteins. However, using UV light to inactivate exosome RNA in vitro only partially impaired their ability to induce regulatory T cells in mouse splenocyte cultures.
Mohammadi explains that each of the molecules identified could have a wide variety of effects, and work in combination, making it tricky for the team to pinpoint the key players for future enhancement of the treatment.
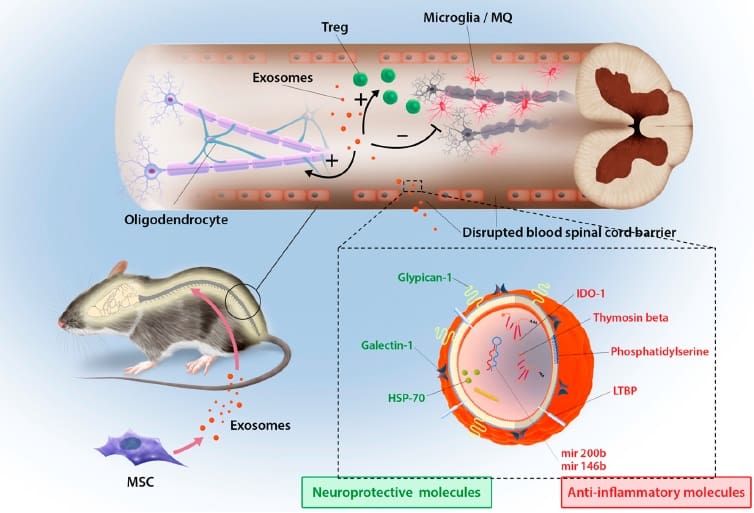
However, they were excited to identify a potential candidate to explain the increased neuroprotection mediated by interferon-gamma stimulated exosomes. “We observed an upregulation in IDO mRNA and protein – an enzyme potent in inducing T regulatory cells and blocking inflammatory T cell differentiation,” says Mohammadi.
Exosomes vs whole cell therapies
The clinical improvement observed in exosome treated mice was similar in stem cell-treated controls, perhaps indicating that exosomes are the main mediators of stem cell’s immune-regulation and neuroprotection in the spinal cord.
By labelling the exosomes with a dye, the researchers were able to identify that at three hours post injection into EAE mice, some exosomes were located in the inflamed spinal cord. The scientists aren’t yet sure how the exosomes’ targeting system works, although they suspect that inflammation acts as the homing beacon, as no accumulation in the spinal cord was observed in healthy mice.
This work has started to answer some of the burning questions in stem cell therapeutics, but many remain, not least of which is whether nanoparticles will prove more efficacious in human autoimmune disease than whole stem cells. Co-author of the study, Milad Riazifar, will try to find out in the first cell-free exosome clinical trial, scheduled for type I diabetes sufferers in 2020.