“Enzyme cascades” are important for biocatalytic transformations in biological cells. These processes involve complex networks and take place in spatially confined microenvironments. A team of researchers at the Hebrew University of Jerusalem in Israel has now made such cascades in the lab by encapsulating three enzymes and enzyme cofactors in nanoreactors made from metal-organic framework nanoparticles (NMOFs). The nanoreactors could be used in a variety of biotechnological applications, such as the catalytic reduction of industrially important chemicals to the development of new and efficient solar energy conversion systems.
“We have shown that the three enzymes can ‘inter-communicate’ in the confined porous structure of the metal-organic framework nanoparticles to the extent that the product of one enzyme can be used as a substrate for the subsequent enzyme to yield a three-enzyme cascade,” explains team leader Itamar Willner. “This mechanism eliminates and overcomes diffusion limitations present in bulk, non-confined, dilute solutions. As a result, biocatalytic transformations occurring in the reactor are very efficient compared to non-confined biocatalytic environments, which means that much lower concentrations of enzymatic catalysts (which are expensive) are needed.”
And that is not all: the encapsulated enzymes in the nanoreactor are stable and not easily denatured. They can also be recycled by the NMOFs matrices. “These features make the biomaterial-loaded nanoreactors ideal cost-effective materials for biotechnological applications,” says Willner.
ZIF8-NMOFs
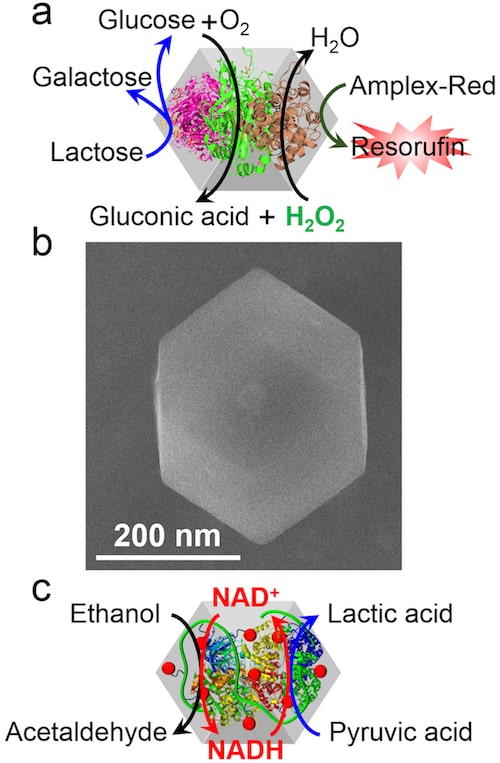
The researchers made their nanoreactor from zeolitic imidazolate framework-8 metal-organic framework nanoparticles (ZIF8-NMOFs). They did this by mixing Zn2+ions with methyl imidazolate at room temperature. In the presence of the enzymes, this technique produces a self-organized porous network on Zn2+ ions cross-linked by the methyl imidazolate ligand in which the biomaterials are encapsulated in the pores and the network matrices exist as highly stable nanoparticles.
“This technique is a versatile approach that can be applied to many other types of biocatalytic cascades that could benefit from being encapsulated in porous confined environments,” Willner tells Physics World. “One example: encapsulating photosynthetic reactions coupled to electron cascades could lead to new efficient solar energy conversion systems.”
In this study, which is detailed in Nature Catalysis s41929-018-0117-2, the researchers encapsulated alcohol dehydrogenase, the cofactor nicotinamide adenine dinucleotide (NAD+) and lactate dehydrogenase in the NMOFs to make a coupled biocatalytic cascade. The enzymes in the cascade catalytically reduce pyruvic acid to lactic acid by ethanol.
“Normally, the two enzymes are made to inter-communicate by the continuous regeneration and recycling of the cofactor,” explains Willner. “This process is inefficient in a bulk solution and is a major obstacle that prevents these systems from being used for biotechnological applications. Confining the enzymes and the cofactor in the porous nanoparticles overcomes this challenge because it produces stable, reusable catalytic nanoparticles.
“Researchers are devoting much attention these days to making catalytic nanoparticles that mimic enzyme functions,” he adds. “These ‘nanozenymes’ and native enzymes in NMOFs could be used to make novel microreactor systems for operating catalytic cascades, and our laboratory is now busy with experiments to make such reactors.”