The recent chance discovery that magnesium diboride becomes superconducting at 40 K - almost twice the temperature of other simple intermetallic compounds - has sparked a race to uncover its basic properties and to process the material for applications.

Superconductivity – the complete loss of electrical resistance in certain metals when cooled to low temperatures – continues to hold surprises. Hot on the heels of the discovery of charge-induced superconductivity at 50 K on the surface of carbon-60 molecules late last year comes the discovery of bulk superconductivity at temperatures approaching 40 K in magnesium diboride. This almost doubles the previous record transition temperature, Tc, of simple intermetallic compounds – previously held by niobium germanium at 23.2 K. This record had not been challenged since the early 1970s, although in 1994 a much more complicated compound – yttrium palladium borocarbide – was found to have a Tc of about 23 K.
In bulk materials the transition temperature of magnesium diboride (MgB2) is only exceeded by the much more complicated perovskite cuprate structures. The new discovery provides a salutary reminder of the richness of the solid state and the way that interesting physics can emerge from quite unexpected areas. Who would have thought of looking for superconductivity in single crystals made of carbon-60, polymers or DNA molecules?
Chance breakthrough
The discovery of this latest superconductor was even more serendipitous than the discovery of the cuprate superconductors by Georg Bednorz and Alex Müller in 1986, for which they shared the Nobel prize a year later. Jun Akimitsu and co-workers at Aoyama-Gakuin University in Tokyo were not even searching for new superconductors when they discovered, quite by chance, that MgB2 loses its electrical resistance. At the time Akimitsu and co-workers were apparently characterizing materials to enhance the current-carrying properties of high-temperature superconductors – compounds that superconduct above the boiling point of liquid nitrogen, 77 K.
The discovery was announced in January at the Symposium on Transition Metal Oxides in Sendai, Japan. Many of those attending the conference immediately rushed home to confirm the result, exciting a major flurry of research on this new superconductor around the world (Physics World March p8, print version only). Akimitsu’s results have now been published (J Nagamatsu et al. 2001 Nature 410 63).
In less than two months since the first announcement, almost 50 papers on MgB2have appeared on the Los Alamos preprint server. The Internet has finally come of age for superconductivity researchers. Anyone can share in the excitement of our rapidly emerging understanding of this new material by simply downloading the latest results. Not all the preprints are high quality, but most add something to the quickly developing picture.
The very rapid progress in understanding and applying this new material is due to the investment in research on the cuprate superconductors over the last 12 years or so. It is almost certain that everything that we need to know about MgB2 and its closely-related compounds will be published in the next six months.
Shelved for decades
One of the most bizarre aspects of this latest discovery is that magnesium diboride has been sitting on chemists’ shelves for almost 50 years. No one recognized that it was even an interesting metal – let alone a record-breaking superconductor. Many of the first measurements, including those made by our group at Birmingham, have simply used MgB2 powder straight from the bottle. Higher-quality materials can be synthesized by mixing, heating and sintering fine boron and magnesium powders together at around 950 oC – preferably under pressure.
Many groups are now trying to grow single crystals for more detailed scientific studies, thin films and superconducting junctions for device applications, and wires and tapes for superconducting magnets and power applications. Indeed, Paul Canfield’s group at the Ames Laboratory at Iowa State University in the US has recently reported the first MgB2wires (see xxx.lanl.gov/abs/cond-mat/0102289). These were made by simply exposing boron wires to magnesium vapour at high temperatures. This is a highly promising route for coating both the inner and outer surfaces of devices, such as filters and cavities for microwave communications and particle accelerators.
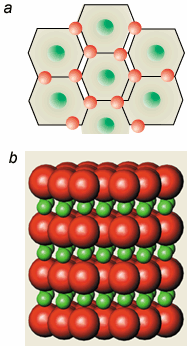
MgB2has a very simple structure in which the boron atoms are arranged in graphite-like planes with magnesium atoms at the centres of the honeycomb cells formed by the boron atoms (see figure). First-principles calculations of the electronic properties have recently been reported by Larry Boyer and colleagues at the Naval Research Laboratories in Washington and the Ames Laboratory (xxx.lanl.gov/abs/cond-mat/0101446). These calculations confirm that the magnesium atoms donate their outer two valence electrons to the strong covalently bonded network of boron atoms to form what is essentially “metallic” boron. Indeed, MgB2is closely analogous to the long sought after, but elusive, superconducting state of metallic hydrogen.
A traditional explanation
Unlike the high-temperature cuprate superconductors, early measurements suggest that MgB2 is a fairly conventional superconductor – albeit with an unexpectedly high transition temperature. In 1955 John Bardeen, Leon Cooper and Bob Schrieffer showed how interactions between the conducting electrons and the vibrational modes of the crystal lattice (phonons) could lead to a pairing of electrons and bulk superconductivity. The three received the 1972 Nobel Prize for Physics for their work.
They predicted that the transition temperature is proportional to the product of the average phonon frequency and an exponential factor that depends on both the number of conduction electrons and the strength of their interactions with the lattice vibrations. The superconductivity of MgB2 appears to be governed by the Bardeen-Cooper-Schrieffer (BCS) theory. The high transition temperature is thought to be due to the high vibrational frequencies of the light boron atoms, and the strong interaction between the electrons and the lattice vibrations.
Within a few weeks of the first discovery, Canfield and co-workers at Ames reported a significant increase (about 1 K) in the transition temperature when the boron-11 atoms were replaced by the less-abundant isotope boron-10. This is fairly convincing evidence for the importance of boron-related lattice vibrations (S L Bud’ko et al. 2001 Phys. Rev. Lett. 86 1877). More recently, several groups have reported that the current-voltage characteristics of electrons tunnelling into the surface of grains are almost exactly as expected from the BCS theory. Meanwhile, a consortium of Japanese researchers has shown that nuclear magnetic-relaxation measurements are also consistent with conventional BCS behaviour.
Superconductivity always involves a delicate balance between the electronic and structural properties of a material. Varying the number of electrons donated to the boron conduction bands can dramatically affect the transition temperature. Indeed, Bob Cava and co-workers at Princeton University in the US have recently shown that the transition temperature of the compound falls if some of the magnesium atoms in MgB2 are replaced by aluminium (xxx.lanl.gov/abs/cond-mat/0102262). A group at the Chinese Academy of Sciences claims to have increased Tc by more than 5 K by doping MgB2 with copper. This has yet to be confirmed by other groups, but doping will certainly allow Tc to be tweaked a little.
High hopes for high supercurrents
Many groups have shown that densely sintered materials can carry very high currents, well in excess of 105 A cm-2 at low temperatures. This is a hundred times greater than the typical current densities in a 13 A copper cable. However, like all superconductors, the transition temperature and maximum superconducting current in MgB2 are seriously degraded in strong magnetic fields. Indeed, superconductivity is completely destroyed in fields of about 15 tesla.
Fortunately, the degradation of superconducting currents by magnetic fields is much less of a problem than it was for the early high-temperature cuprate superconductors. We can confidently expect major improvements in the maximum current that can be carried by MgB2 as materials scientists learn to optimize the superconducting properties. Currently, conventional superconductors like niobium tin and the bismuth-based cuprate superconductors easily outperform MgB2at the same temperatures. But this situation might be reversed if the electronic mean free path can be significantly shortened without changing any of the other properties significantly.
Cooling superconducting wires, cavities or devices to about 30 K, so that they operate well below the transition temperature, should not be a technological problem. Such temperatures can easily be reached without the need for liquid helium or nitrogen by using compact, and relatively inexpensive, mains-driven cryocoolers.
Why did it take so long?
Many people ask why superconductivity in such a simple compound as MgB2was not identified for almost 50 years. In the early 1950s, prior to BCS theory, Bert Matthias and John Hulm at the University of Chicago depended on empirical rules to guide them in their pioneering search for new superconductors. They were among the first to consider the wide range of simple intermetallic compounds containing non-metallic elements such as boron, carbon, sulphur and nitrogen.
Higher-temperature superconductors – such as niobium nitride, niobium carbide, niobium boride and molybdenum diboride with transition temperatures ranging from 4.74 – 14.7 K – were almost exclusively based on transition metals. It was also known that increasing the amount of boron in intermetallic compounds resulted in more strongly covalently bonded network structures, which were expected to be poorer conductors. Indeed, Matthias and Hulm were unable to discover superconductivity among any of the transition-metal diborides they investigated. Their empirical rules implied that there would be even less chance of finding superconductivity in a compound like magnesium diboride.
It is therefore sensible to ask whether there might be other binary and ternary intermetallic superconductors waiting to escape from the chemists’ shelves. Some groups are now testing all the black-looking powders they can find for potential superconductivity. Keep watching this space!