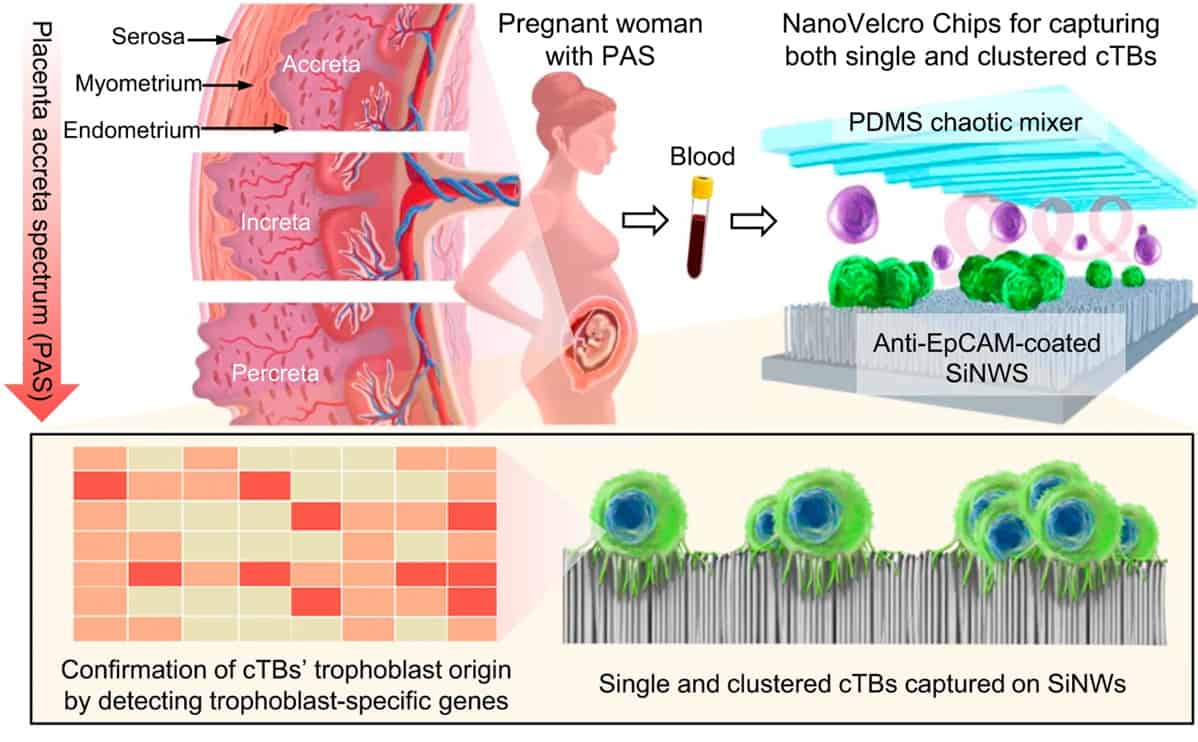
Placenta accreta spectrum (PAS) is a high-risk pregnancy complication that occurs when the placenta is not able to naturally separate from the mother’s uterus after delivery. The undetached placenta must then be removed through clinical intervention, which can lead to life-threatening issues such as severe haemorrhage. PAS currently occurs in 1 in 500 pregnant women; however, this proportion is increasing worldwide due to the rising rates of caesarean delivery.
To improve maternal outcomes after delivery, there is a need for a sensitive and accurate tool that can detect PAS during the early stages of pregnancy, enabling the disorder to be effectively managed by an interdisciplinary clinical team. Recently, an international team, led by researchers at the University of California, Los Angeles (UCLA) and Cedars-Sinai in Los Angeles, has assessed a non-invasive cell-capturing device called the NanoVelcro Chip, which aims to provide early detection of PAS. The team published the results of this study in Nature Communications.
“Early and precise detection of this very high-risk obstetrical problem can greatly improve outcomes for both the mother and baby,” says co-first author Yalda Afshar from UCLA in a press statement. “With the unreliability of the current screening methods for placenta accreta, we saw a pressing need to create easy-to-implement screening that can be conducted early in the pregnancy in all healthcare settings regardless of resources available to patients.”
Selective cell capture
During a normal pregnancy, trophoblast cells adhere to the uterine lining and aid in the development of the placenta. These placental trophoblasts are then shed from the uterus and enter the maternal bloodstream. However, an error can occur during this process that causes an abnormally high number of trophoblasts to attach to the uterine lining. This can lead to an increased number of circulating trophoblasts within the blood, indicative of an elevated risk of PAS. The NanoVelcro Chips are therefore designed to non-invasively detect quantitative changes in these circulating trophoblasts within the blood of pregnant women.
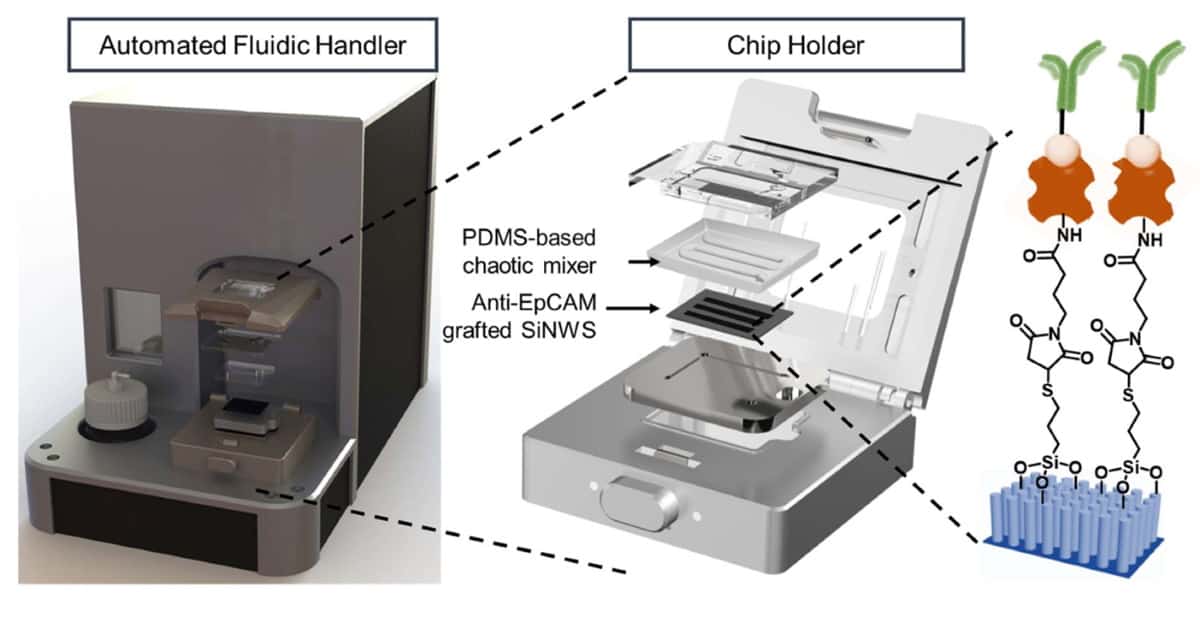
The NanoVelcro Chip is held in a device that distributes the blood sample throughout the microchip’s two functional components. The top component contains a layer of polymer-based chaotic mixer, which promotes the frequency of contact between the blood cells and the microchip’s nanostructure.
The bottom component contains a silicon nanowire substrate coated with a molecule-adhesion layer. This layer contains antibodies that specifically recognize single and clustered circulating trophoblasts, while the nanowires function to lock up these cells within the microchip. The number of circulating trophoblasts captured by the NanoVelcro Chip determines the risk of having PAS disorder.
Detecting PAS disorder
In essence, NanoVelcro Chips act like biological Velcro strips that have been specifically designed to entangle single and clustered circulating trophoblasts in the mother’s bloodstream. To determine the efficacy of this novel microchip, the researchers used the NanoVelcro Chip device to analyse blood samples from 168 pregnant women diagnosed with PAS disorder and 15 healthy non-pregnant females.
The results of this pilot study showed that the detection rates of these circulating trophoblasts were significantly higher in the pregnant women with PAS than in the healthy control group. These findings were validated through the examination of placental tissues biopsied from PAS patients. Further validation using immunocytochemistry ensured that the cells captured on the microchip were indeed single and clustered circulating trophoblasts.
Ultimately, this novel microchip device provides the potential to aid in the early detection of PAS. “Timely detection and diagnosis of PAS provide opportunities to improve prenatal care and minimize maternal and neonatal morbidity,” the authors conclude. “Our study demonstrates a promising non-invasive technology for detecting PAS that does not rely on imaging instruments or expertise by taking advantage of the in vitro diagnostic value of NanoVelcro Chips.”



