Physicists in Switzerland have observed a novel type of molecule known as an exciplex - short for excited complex - for the first time. Antoine Weis, Daniel Nettels, Peter Moroshkin and colleagues at the University of Fribourg have seen evidence for an exciplex that contains one caesium atom and seven helium atoms, as well as a simpler version that contains just two helium atoms (D Nettels et al. 2005 Phys. Rev. Lett. 94 063001). Until recently it was thought that exciplexes containing more than two helium atoms could not exist.

Exciplexes are molecules that can only exist if one of the atoms in the molecule is in an excited state. If this atom returns to its ground state the exciplex falls apart. For example, alkali atoms and helium atoms strongly repel each other in their ground state at short distances because of the Pauli exclusion principle. However, if the alkali atom is excited by a laser, for example, the force between the atoms becomes attractive and an exciplex molecule can form.
The first alkali-helium exciplexes were observed in 1995, and since then they have been seen in liquid helium, cold helium gas and on the surface of helium nanodroplets. Now, the Fribourg team has detected exciplexes in solid helium for the first time.
Weis and co-workers began by doping a solid helium-4 matrix with caesium atoms at a temperature of 1.5 Kelvin and a pressure of 31.6 bar. Next, they excited the embedded atoms with a laser and recorded the fluorescence emission spectrum from the sample. The Fribourg team observed two new features in the far infrared region of their plot (figure 1).
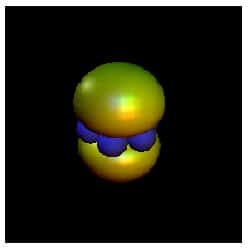
One of these features could be attributed to an “apple-shaped” exciplex containing two helium atoms and one excited, and much larger, caesium atom. This molecule has also been seen in liquid helium and cold helium gas. However, a second feature could only be explained in terms of a exciplex shaped like a dumbbell that contains seven helium atoms arranged on a ring around the caesium atom (figure 2).
“I find it exciting that the newly discovered molecule may exist only in very special conditions, at extremely low temperature and high pressure,” says Moroshkin. “Molecules of this kind are interesting objects for studying fundamental quantum physics and quantum chemistry.” The team now plans to extend its study to other alkali-helium complexes, such as rubidium-helium.