Helium is an incredibly useful substance, but so far most sources have been discovered by accident. Diveena Danabalan describes how she and her colleagues are working to change that

Helium is the second-most common element in the universe, but here on Earth it is classified as a scarce and non-renewable resource. It is also much in demand, with applications including medical cryogenics (as of 2016, around 30% of helium consumed in the US went to equipment such as MRI scanners), arc welding, leak detection and superconductivity. The combination of scarcity and high demand has pushed prices skyward: the price of grade-A helium (refined to 99.997% purity) has risen by around 175% over the past 10 years, to $7.21/m3, and even crude helium is now worth around $3.75/m3 – 30 times more than the equivalent volume of crude methane.
In many markets, numbers like these would make investors salivate. Yet so far, all helium-rich fields have been discovered accidentally by companies searching for petroleum. This lack of intent has contributed to a great deal of wastage: we know that many natural gas production companies are unwittingly venting helium, either because they fail to recognize the value of this minor component or because they are unaware of its presence. Indeed, our discovery of one such case produced the idea for our current research project.
A helium-prospecting industry powered by people who understand both the economic value of this rare material and how to explore for it. But when oil and gas companies prospect for petroleum, they benefit from a well-developed exploration strategy that helps them assess a new basin or area in a systematic way. Unfortunately, there is currently no equivalent methodology for helium.
My colleagues and I are seeking to address this gap in our knowledge. In a joint research venture between Durham University and the University of Oxford in the UK, with the financial support of the Norwegian state oil company Statoil, we have used well-established hydrocarbon exploration protocols as a template to develop a similar strategy for helium. This work has enabled us to begin to answer questions about how and where helium is generated; how it is released from potential source rocks; how it then travels significant distances from source rocks to potential traps; and how helium-rich gas accumulates in the shallow crust, as well as how these accumulations can be destroyed by natural processes. The project is still in its early stages, but we believe our research is an important step towards diversifying the helium supply, so that when another supply crisis occurs, we are better prepared.
Know your helium
There are two stable isotopes of helium: ubiquitous helium-4, which constitutes 99.999% of helium gas, and rare helium-3. Helium-3 is used in neutron detectors and is also a candidate fuel for power generation though nuclear fusion. It is often referred to as “primordial helium”, since the bulk of it was trapped in the Earth’s mantle during the planet’s formation. The more common isotope, helium-4, is mainly produced by the alpha decay of uranium-235, uranium-238 and thorium-232 in the Earth’s crust, which has led to it being called “radiogenic helium”.
Different rock types produce varying amounts of helium-4, controlled by the original concentrations of uranium and thorium and the age of the rock. Some of the highest accumulations of helium are found in large, stable continental blocks known as cratons that formed in the early Precambrian (up to 4.28 billion years ago), such as the Canadian Shield. Helium is found in many other geological systems, including groundwater, ancient brines, fluid inclusions in ore deposits, hydrothermal fluids, igneous intrusions and rocks, oil-field brines, lakes, ice sheets, oceanic sediments and coal measures. To date, however, only a few natural hydrocarbon gas fields contain helium in sufficient concentrations for its extraction to be considered commercially viable.
The first economic quantities of helium were discovered in 1903 in Dexter, Kansas, US. Analyses of this gas – which was initially referred to as “wind gas” because it was non-flammable – found that it contained approximately 82.7% nitrogen and up to 1.84% helium by volume. This concentration of helium was unusually high, as most natural gas fields contain helium only in trace amounts (≤0.05%). Significant discoveries in eight other US states soon followed, with some of these fields containing up to 10% helium by volume – significantly above the threshold at which helium is considered economically extractable (0.3%). Helium reserves have also been found in Algeria, Canada, China, Germany, Hungary, India, Kazakhstan, Pakistan, Poland, Qatar, Romania, Russia and the Timor Sea. However, none of these new helium reserves currently match the concentrations, number of occurrences or estimated reserve volumes associated with the older US fields.
Finding new sources
From a simplistic sourcing perspective, older rocks will generally have had more time to produce and accumulate helium than younger ones. Therefore, a logical first step in a helium exploration protocol is to identify viable Precambrian-aged crystalline terrains in areas that have remained relatively tectonically stable for long periods of time. However, age is not the sole determinant of a viable source rock. The helium also needs to have been released from the rock – a process known as primary migration.
The primary migration process begins when particles in uranium and thorium-bearing minerals undergo radioactive decay, releasing alpha particles (helium-4 nuclei). The energy associated with this process produces “fission tracks” through the mineral, and helium atoms can readily diffuse along these tracks. While it is not yet known how efficient this escape process is, once helium has diffused out of the mineral it will accumulate in fluid inclusions and fractures within the source rock. For helium to then migrate out of the low-permeability source rocks into overlying layers, another input of energy is required. This typically comes in the form of heat and pressure from tectonic events such as rifting, mountain building or volcanic activity.
We do not fully understand the primary release process; however, we do know that the bulk migration of helium is enhanced by the presence of a carrier fluid or gas. In natural gas formations, high helium concentrations are always associated with high concentrations of nitrogen. The converse is not true, as many nitrogen-rich natural gas sources contain only trace amounts of helium; the explanation being that there are multiple sources of trapped nitrogen in the crust. Analyses have shown that radiogenic helium is consistently associated with nitrogen that has an isotope distribution characteristic of a source in the crystalline basement, indicating that the nitrogen is likely carrying helium out of source rocks.
Early in our research, we sampled well gases from existing helium-producing areas in the midwestern US and southern Canada. More recently, we worked with a helium exploration company, Helium One, to sample helium-rich gas seeps in the Tanzanian section of the East African Rift. All samples were rigorously analysed for information about the gas composition and the isotopic composition of the separated helium, other noble gases and nitrogen. This gave us an idea of the interaction between helium and its associated nitrogen carrier, and how they migrate out of source rocks. However, we still needed to account for the way in which helium and nitrogen moves (sometimes hundreds of kilometres) and collects into geological “traps” – a process known as secondary migration.
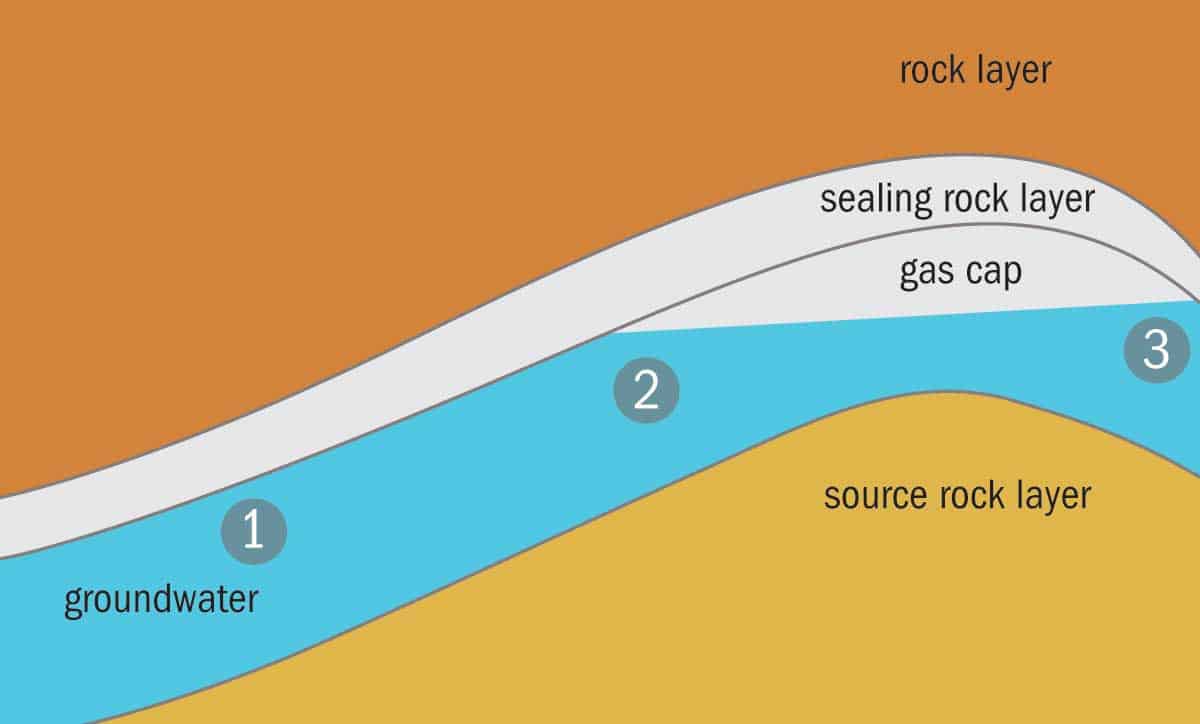
Our studies indicate that once helium and nitrogen are released from the source rock, they interact with groundwater in overlying strata (see figure above). Once enough helium and nitrogen are dissolved in the groundwater, they are able to form a separate nitrogen- and-helium-rich gas phase as the groundwater ascends to the surface and becomes depressurized. We think this is the mechanism for the near-pure nitrogen-helium gas fields found in North America.
Other helium-producing fields have a more complex makeup of gases. Some (such as LaBarge/Riley Ridge and Doe Canyon in the US) are known to contain primarily carbon dioxide, and others (like the North Dome in Qatar, Hassi R’Mel in Algeria and the Hugoton-Panhandle in the US) are rich in methane. The presence of significant concentrations of carbon dioxide or methane in these helium-rich natural-gas trapping structures gives us another clue as to the possible mechanisms behind helium trapping. If groundwater containing dissolved helium and nitrogen comes into contact with a pre-existing natural gas cap then helium and nitrogen will preferentially move from the groundwater into the gas cap.
But there is a complication. Because carbon dioxide and methane are both prevalent in the subsurface, there is a high risk that in locations where helium and nitrogen accumulate, the helium concentration may be diluted by large amounts of these other gases – potentially to levels that are not worth extracting commercially. In formations where we are relying on methane and carbon dioxide to strip dissolved helium and nitrogen, we need to establish a zone where the degree of dilution is “just right”. As an example, volcanic activity is a known source of high-carbon-dioxide gas fields. Therefore, in the right geological setting, the thermal aureole associated with magma emplacement may provide the heating needed to release helium from its source. However, if the trap is too close to the volcanic centre, the only gas you are likely to find there is carbon dioxide – whereas further from the volcanic source you are more likely to see nitrogen-rich helium gases like the ones that occur in the Tanzanian seeps we sampled.
Once helium has migrated into a gas-trapping structure, the preservation of helium in that trap depends on the rate at which helium is supplied to the deposit and the efficiency of the seal or trap to contain the gases. Trap destruction (caused by weathering, erosion or tectonic events) or a leaky seal (usually caused by the pressure in the reservoir exceeding the pressure in the overlying caprock) will result in helium being lost from the trap.
Next steps for helium exploration
Based on this preliminary helium exploration methodology, we have identified the Tanzanian region of the East African Rift as a potential helium-rich system. The region contains an ancient craton that has been perturbed by a much younger rifting event and, as a bonus, some of the gas seeps in the region are already known to be nitrogen-helium rich.
But our database and geochemical analyses of the helium-rich fields are lacking, especially when compared with the years of accumulated data for petroleum exploration. Continuing to sample and analyse natural helium occurrences so as to better understand and classify migration and accumulation processes is essential. Nevertheless, we believe we have taken an important step towards securing the future of our helium supply.
- Enjoy the rest of the 2017 Physics World Focus on Vacuum and Instruments in our digital magazine or via the Physics World app for any iOS or Android smartphone or tablet.