Optical scintillation imaging is proving feasible as a quality assurance (QA) tool for small static beams and for pre-treatment verification of radiosurgery and volumetric-modulated arc therapy (VMAT) plans. Researchers at Dartmouth College have shown that the technique can perform QA for each control point within five dynamic VMAT plans. Moreover, the approach is sensitive to small errors in gantry angle and multileaf collimator (MLC) leaf position (Med. Phys. 10.1002/mp.13797).
Recently, Brian Pogue and colleagues at the Thayer School of Engineering successfully used Cherenkov imaging as a QA tool for broad beams from an MR-linac. They demonstrated good agreement between optical images acquired from an intensified CCD camera and simulated dose distributions from the treatment planning system (TPS), but were not able to image small beamlets reliably due to a low signal-to-noise ratio.
“While the detection principle is the same as Cherenkov imaging, in scintillation imaging, an organic phosphor is used to boost the signal, allowing the recording of the smallest, dynamic beams with high fidelity,” explains co-author Petra Bruza.
In their latest study, the researchers used a unique water-based scintillator, which minimizes optical blurring and provides high optical signal while being fully tissue (water) equivalent. They acquired data with a blue-sensitive intensified CMOS camera, which captures optical photons generated due to ionizing radiation incident on a cylindrical water phantom containing quinine sulphate.
“The camera contains an optical intensifier that is remotely synchronized with the X-ray beam,” Bruza tells Physics World. “Optical amplification greatly improves the signal from small beamlets, while the remote trigger suppresses background and also provides additional information about the beam stability.”
The team compared projected percentage depth dose and cross beam profiles for static 6 MV beams of various widths with data from the TPS. The average differences between the TPS and optical depth dose profiles were 0.52%, 1.25% and 1.53%, respectively, for 50, 10 and 5 mm beam widths. All cross beam profiles exhibited a 3%/1 mm gamma passing rate of greater than 99%.
Next, the researchers delivered five clinically approved VMAT plans to the water phantom, including a prostate plan, two head-and-neck plans, a stereotactic body radiation therapy plan and a multi-lesion stereotactic radiosurgery (SRS) plan. Representing a range of different VMAT plan types with varying degrees of modulation and field sizes, these had dose rates of 120 to 2400 MU/min.
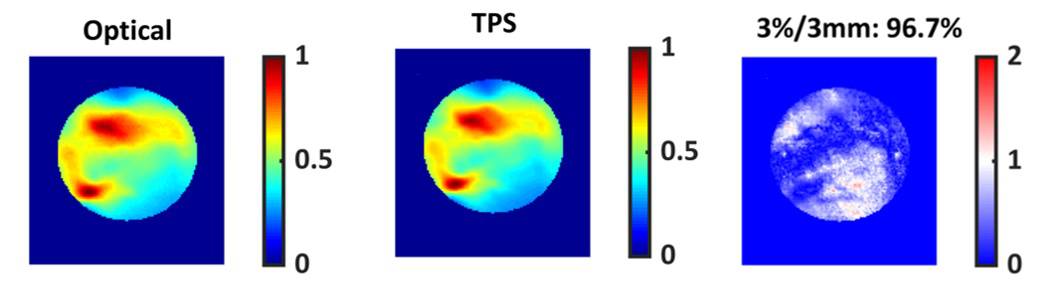
All five plans passed the 3%/3 mm gamma criteria. The technique achieved good agreement between the TPS-simulated dose and the optical images, with an average gamma pass rate of 97.8%. The technique was sensitive to MLC errors down to 1 mm and gantry angle errors of 1°, and achieved a spatial resolution of 1 mm.
“The technique was found to be more sensitive than the diode array for common delivery errors,” says co-author Muhammad Ramish Ashraf. “Moreover, it was found that the technique was particularly sensitive to overmodulated plans.”
“A key advantage of optical scintillation imaging is that it records dose from the target treatment volume inside the phantom, where the quality assurance is most important,” explains Bruza. “It does it with true submillimetre lateral resolution. Some of the most advanced other approaches record dose either from the ‘skin’ of the phantom, or from a single plane inside the phantom, both with much coarser resolution of 5 to 10 mm.”
He notes that optical scintillation imaging also has advantage for MRI-linac systems. As it places all of the electronics outside the phantom, it further minimizing interference between the dosimeter and MRI.
Novel takes on MR-linac dosimetry
The team’s next project is to improve detection of the X-ray cross-beam profile to enable seamless 3D imaging and to extend the technique to proton therapy QA. “Our research is focused on further refinement of our 4D (3D plus time) dose reconstruction project and translating it into clinics,” says Bruza. “By using additional information about X-ray cross-beam section, we can reconstruct 3D dose with submillimetre resolution and temporally with at least 20 frames per second frame rate.”
Cumulating these volumes in time provides the final 3D dose distribution. “With that, we can check how the dose truly matches the tumour shape, and how it avoids organs-at-risk, in the form of a dose–volume histogram,” says Bruza. “The dose–volume histogram is the best way how to represent the quality of dose delivery, because it relates the dose distribution to the actual clinical structures.”




