Advances in imaging technology and screening programmes have enabled earlier detection of breast cancer, allowing the use of non-invasive treatments such as high-intensity focused ultrasound (HIFU). HIFU can ablate small regions without damaging adjacent tissues and is also repeatable.
During HIFU, ultrasound emitted from a transducer outside the body propagates through tissue to focus on the target. This focused acoustic energy raises the temperature in the target to necrosis-inducing levels within a few seconds. HIFU has been used previously for breast cancer treatment, but its success rate varies widely, from 20 to 100%, depending upon factors including the system type, imaging technique, ablation protocol and patient selection.
To optimize breast cancer HIFU, a Japanese collaboration has used numerical simulations to determine the relationship between breast tissue structure and focal error, which is caused by reflection and refraction of the ultrasound wave due to the acoustic inhomogeneity of the body. Improving ultrasound focusing should result in more efficient and safer treatments (J. Therapeutic Ultrasound 10.1186/s40349-018-0111-9).
HIFU modelling
The research team – headed up at Nihon University and the University of Tokyo – used MRI data from 12 patients to create digital breast phantoms comprised of skin, fat and fibroglandular tissue. These phantoms formed the input data for the simulator ZZ-HIFU, which simulates ultrasound propagation through tissues with varying acoustic properties.
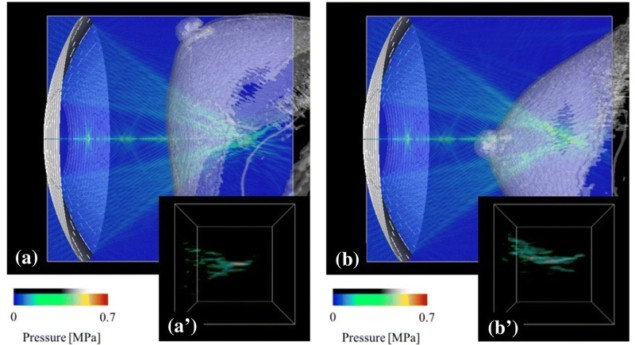
The researchers examined the impact of different target positions and transducer arrangements on the focal error. To evaluate focal error, they determined the focusing ratio for various breast phantoms and set-ups. They simulated ultrasound wave propagation from a 256-channel phased-array transducer through the skin to a target, with the acoustic axis passing either through breast tissue only (A) or through the nipple (B).
The focal shape, a high-pressure amplitude region around the focus, was distorted as the ultrasound waves propagated through the breast tissue. Arrangement A led to less distortion, with a focusing ratio of 0.093, compared with 0.094 for B. As these values are considerably lower than obtained without breast tissue (0.243), the researchers normalized the results to this value. The normalized focusing ratios were 0.384 and 0.386, for A and B, respectively.
They then applied a focus control technique, based on a time-reversal method, to the simulation of arrangement B. This process provided an ultrasound wave correctly focused on the target with a clearer focal shape, and considerably improved the normalized focusing ratio, from 0.386 to 0.543.
Using a different breast phantom, the researchers compared the relationship between fibroglandular structures and focal shapes in the two target arrangements. Again, the focal shape for A was much clearer than that for B. In the former, no fibroglandular tissue was observed between the skin and focus, whereas in the latter, the focus was deep in the fibroglandular tissue and highly distorted. The normalized focusing ratios in these two cases were 0.901 and 0.465, respectively.
The team also examined the impact of the brightness threshold used to segment fat and fibroglandular tissue. They found that higher thresholds (indicating more fatty tissue) led to lower focusing ratios and increased focal distortion.
Optimizing treatments
The simulations showed that focal error was caused by the complex distribution of fibroglandular tissue and was dependent on the target position and transducer arrangement. As such, the authors suggest arranging the HIFU transducer such that the ultrasound wave avoids fibroglandular tissue.
If a tumour is located deep in fibroglandular tissue, focus control using the phased-array transducer is required for safe and efficient treatment. The researchers showed that focusing ratios can be increased using focus control based on the time-reversal method. They point out that, as the focusing ratio increases with a decrease in local acoustic inhomogeneity, it may be used as an indicator to reduce the HIFU focal error depending on the breast structure.
“The obtained results demonstrated that the focal error observed during the breast cancer HIFU treatment is highly dependent on the structure of fibroglandular tissue,” the authors conclude. “The optimal arrangement of the transducer to the target can be obtained by minimizing the local acoustic inhomogeneity before the breast cancer HIFU treatment.”