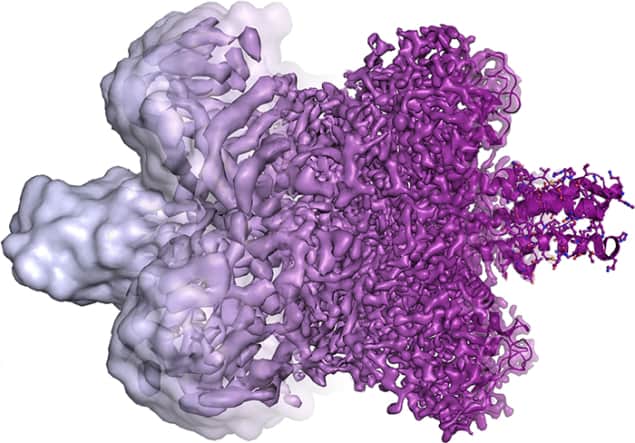
The 2017 Nobel Prize for Chemistry has been given to Jacques Dubochet, Joachim Frank and Richard Henderson “for developing cryo-electron microscopy for the high-resolution structure determination of biomolecules in solution”. The prize is worth SEK 9m (£823,000) and will be presented at a ceremony on 10 December in Stockholm. It is shared equally by the three laureates, who are all physicists by training.
Cryo-electron microscopy gets around two major challenges when studying large biological molecules with a transmission electron microscope. First, the molecules exist naturally in water, the vapour from which destroys the vacuum required to run the microscope. The molecules could be dried out before being examined, but this can alter their structure so much that the study would be pointless. The second challenge is that the electron beam heats up and destroys delicate biological molecules. This can be addressed by using a weaker beam, but that would result in fuzzier images.
Henderson showed in the early 1970s that a protein called bacteriorhodopsin can be studied under an electron microscope if the molecules are naturally bound within a biological membrane. This keeps the molecules wet and stops water from evaporating into the vacuum. To minimize destruction by heating, Henderson exploited the fact that the bacteriorhodopsin molecules in the membrane are arranged in a regular array, which lets him use a weak beam of electrons to build up a diffraction pattern. He obtained the protein’s structure in 1975.
Atomic-scale images
As electron microscopes improved, Henderson was eventually able to deduce the structure at the atomic scale by 1990 – showing that it was possible to do high-quality studies of biological molecules using an electron microscope.
Working independently in 1975, Frank unveiled an image-analysis algorithm for merging multiple fuzzy 2D images from an electron microscope to create a 3D image of a molecule. This involves taking thousands of images of randomly oriented molecules and sorting them into groups of similar images. Each group is then processed to create a set of much sharper images. The spatial relationships between the groups are then calculated, leading to the assembly of a high-resolution 3D image.
It turned out that Frank’s algorithm could be directly applied to a technique for imaging biological molecules that had been developed independently in 1982 by Dubochet. This involves dispersing molecules in a thin film of water suspended in the gaps of a mesh. The water is then frozen rapidly by plunging the mesh into liquid ethane at –190 °C. Rapid freezing means that the water molecules in the ice do not have a regular crystal structure, but instead resemble a glass. This “vitrification” of water is crucial because it does not cause electron diffraction and allows images to be taken – albeit fuzzy ones.
Better microscopes
In 1991, Frank combined Dubochet’s vitrification technique with his imaging algorithm to obtain images of ribosomes. As electron microscopes have improved over the intervening years (in part thanks to Henderson’s efforts) cryo-electron microscopy can now image biological molecules at the atomic level.
Born in Scotland in 1945, Henderson did a BSc in physics at the University of Edinburgh before completing a PhD in molecular biology at the University of Cambridge, UK, in 1969. After a three-year stint at Yale University, he joined the Medical Research Council’s Laboratory of Molecular Biology, also in Cambridge, where he is a group leader.
Dubochet was born in 1942 in Switzerland and is based at the University of Lausanne, where he is honorary professor of biophysics. He studied physics and engineering at the École Polytechnique Fédérale de Lausanne before obtaining a PhD in biophysics in 1973 from the University of Geneva and the University of Basel.
Frank was born in 1940 in Germany and studied physics at the University of Freiburg and the University of Munich. He completed a PhD on electron microscopy in 1970 at the Technical University of Munich. He later worked at several universities and research labs worldwide before joining Columbia University as professor of biochemistry and molecular biophysics and of biological sciences in 2008.