Non-alcoholic fatty liver disease (NAFLD) affects over one billion people worldwide. However, patients are typically asymptomatic in the early stages of the disease. This means that NAFLD often remains undetected until it progresses to non-alcoholic steatohepatitis (NASH), a far more aggressive condition that can ultimately lead to liver failure.
While NASH causes permanent liver damage, NAFLD is treatable through lifestyle changes and medication. Creating a diagnostic test that allows for widespread screening could help to identify those at risk of NASH before the damage is too severe.
Researchers from Massachusetts Institute of Technology have developed a portable nuclear magnetic resonance (NMR) sensor that grades liver tissue according to its fat content. Described in Nature Biomedical Engineering, the device tracks water as it diffuses through the liver. The rate of diffusion reveals how much fat is present in the organ, providing a quantitative metric of NAFLD, even in the early stages of disease progression.
Using NMR as a predictor for fatty liver disease
A healthy liver stores little to no fat. By contrast, NAFLD is characterized by abnormal fat retention within liver cells, or steatosis.
Excessive steatosis may trigger NASH, causing inflammation and fibrosis (scarring). The severity of fibrosis can be measured using a biopsy, but this is highly invasive and ineffective if a non-fibrotic portion of the liver is tested.
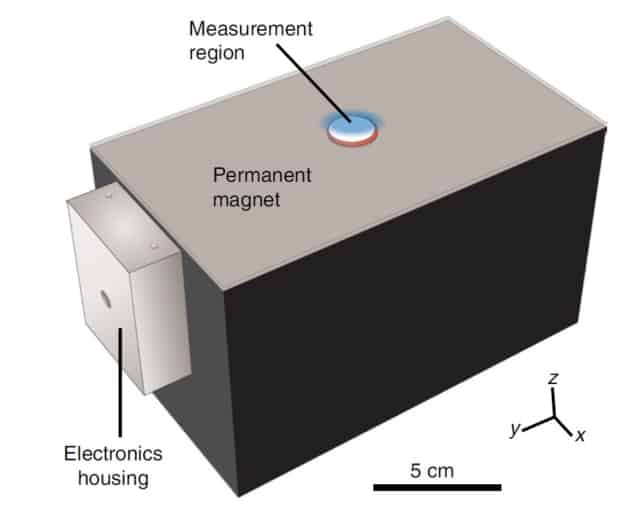
An alternative approach, and the method used by Michael Cima and his team, is to evaluate the progression of NAFLD using NMR. NMR is particularly sensitive to changes in tissue composition caused by steatosis and fibrosis. The compact sensor created by the researchers looks to combine the diagnostic power of traditional (but bulky) MRI scanners with the portability needed for routine NAFLD/NASH screening.
The researchers use a technique called diffusion-weighted multicomponent T2 relaxometry to monitor the magnetic properties of water – specifically its 1H atoms – as it diffuses through the liver. When a sample is placed on the surface of the sensor, the proton spins align with the remote magnetic field generated by the permanent magnet. The protons are subsequently knocked out of alignment using a specific radiofrequency pulse sequence and the resulting transverse magnetization is recorded by the transceiver coil.
This magnetization decays as the water diffuses through the sample. How quickly this relaxation occurs depends upon the degree of steatosis and scarring – water diffuses more slowly through fatty, fibrotic tissue.
Using multiexponential fitting to process the decay signal, the researchers can correlate the relaxation time with the amount of fat contained within the sample.
Potential for point-of-care diagnostics
When testing the sensor in vivo in a diet-induced mouse model for NAFLD, the researchers reported a steatosis grading accuracy of 87%. The results demonstrate how, in principle, the sensor could be used to track the progression of NAFLD to NASH. The team also tested their method on human liver samples and were able to grade steatosis with 93% accuracy.
Currently, the model is limited to a penetration depth of 6 mm. To enable non-invasive imaging of patients, changes to the geometrical design of the sensor are needed. Such changes must, however, be made without compromising the portability, the team warn.
The implications of using the sensor in patients could go beyond staging the onset of NAFLD and NASH. “Having this type of diagnostic available could also aid in drug development efforts, because it could allow doctors to more easily identify patients with fibrosis and monitor their response to new treatments,” says Cima.



