Researchers have succeeded in using the “protein corona” that naturally absorbs onto nanoparticles, such as liposomes, when they are placed in biological fluids to develop an advanced blood analysis technique. The new approach could allow for the identification of biomolecules that hitherto could not be detected by conventional blood plasma proteomic analyses. The nanotool might find applications in a number of areas in nanomedicine, including the early diagnosis of cancer.
Among the many drug delivery systems that exist today, nanoparticles known as liposomes (soft phospholipid-based vesicles) are one of the most advanced. These drug carriers help minimise the toxic side effects of many therapeutics including those used to combat cancer (one example is doxorubicin), while allowing the drugs to remain in the blood stream for longer.
The protein corona
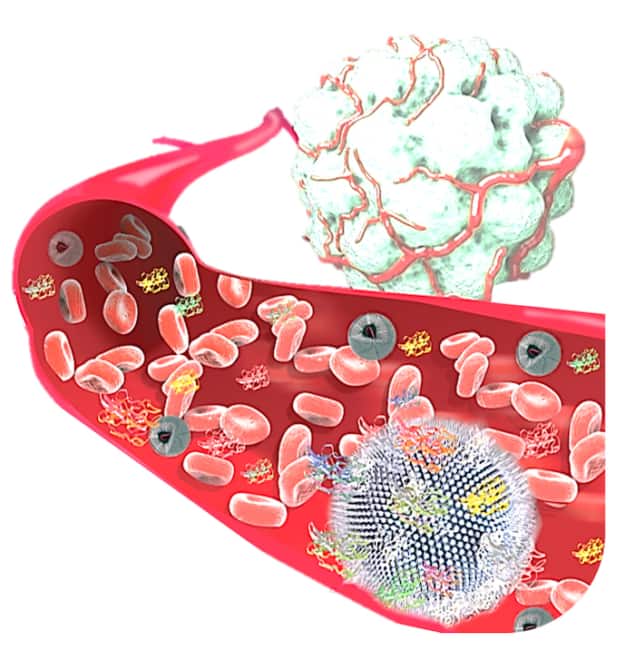
“Despite the good clinical track record of liposomes, researchers have only recently started to pay attention to the role that the protein corona plays in liposome pharmacology,” says Kostas Kostarelos of the University of Manchester in the UK, who led this research effort. This “halo” refers to the spontaneous and layered adsorption of biomolecules onto the liposomes when they are injected into the blood stream and it could provide valuable information on which disease biomarkers a patient is carrying. Usually these markers are too small and present in too low concentrations to be detected.
Kostarelos and colleagues have now analysed blood samples taken from patients with advanced stage ovarian cancer who were treated with the anti-cancer drug CAELYX®, which contains doxorubicin encapsulated in a liposome nanoconstruct. “We recovered the liposomes from the blood circulation of the patients and were able to detect a wide variety of disease-specific proteins adsorbed onto the liposome surface,” explains Kostarelo. We found that the corona was particularly rich in low-molecular weight and low-abundant plasma proteins.”
The researchers analysed the liposomes and their coronas using a number of techniques, including dynamic light scattering, ζ-potential measurements, negative strain transmission electron microscopy and mass spectrometry.
In vivo and ex vivo experiments
The team compared the molecular composition of the protein corona that forms in vivo around intravenously injected CAELYX® liposomes with the ex vivo corona that forms after simply incubating the liposomes with plasma samples taken from the same ovarian carcinoma patients.
“In agreement with our previous data in rodents, we detected a more complex molecular fingerprint for the in vivo protein corona in comparison to its ex vivo counterpart,” says study lead author Marilena Hadjidemetriou. “Despite the fact that we did not detect one particular protein, the cDNA clone CS0DD006YL02 (which has never been described or reported on before) in any of the control plasma samples, we did identify it as the most abundant protein in both the in vivo and the ex vivo formed protein coronas, indicating that the nanoparticle protein corona allows the identification of previously unseen blood molecules.”
Liposome-like nanovesicles target tumours
Kostarelos and colleagues also did the same experiments on a control group of healthy volunteers by incubating blood plasma samples taken from these volunteers with CAELYX® liposomes. They found that the CS0DD006YL02 protein was only the fifth most abundant protein in the ex vivo corona formed in this group. This result suggests that tumorigenesis can be reflected in the molecular composition and dynamics of corona formation, but much more work is needed to confirm this hypothesis, they say.
“In previous work, we showed that protein coronas qualitatively and quantitatively change in tumour-bearing mice, thus allowing detection of differentially abundant marker molecules that distinguish between healthy and diseased states,” explains Kostarelos. “Although the in vivo protein corona allows analysis of a molecularly richer blood fingerprint and can be a valuable tool for biomarker discovery in mouse models, we now working to optimise the ex vivo protein corona analysis for biomarker discovery using human blood samples.”
Detecting early stage ovarian cancer
“We’re astonished at how rich the information was on the surface of the liposomes taken from blood,” he says. “We hope this technique could be a springboard for further research, from monitoring disease progression or recurrence, to identifying which treatment is best for each patient and potentially finding new biomarkers for early diagnosis.”
Blood is a potential goldmine of information but there’s a challenge to amplify cancer signals that would otherwise be buried within the “noise”, adds Hadjidemetriou. “More abundant proteins mask rarer and smaller molecules that could be significant in helping us to understand disease progression or finding potential new drug targets. Our technique overcomes this challenge.”
The researchers, who were funded by Cancer Research UK, are now planning to use their technique to discover the best biomarker patterns for early-stage ovarian cancer. They report their work in Advanced Materials 10.1002/adma.201803335.