Small-animal irradiation studies are an essential component of cancer research, bridging the gap between cell experiments and clinical realization of emerging radiotherapy techniques. But while photon-based preclinical radiation research platforms have been commercialized, the same does not hold true for protons.
The SIRMIO project, established by Katia Parodi at Ludwig-Maximilians-Universität München (LMU), aims to bring together proton therapy and preclinical research by developing a dedicated platform for small-animal proton irradiation. To achieve high-precision placement of the Bragg peak within the target, the team is equipping the platform with a proton CT (pCT) system.
“So far, image guidance on small-animal photon radiation research platforms, which are being adopted for proton therapy, uses X-ray cone-beam CT,” Parodi explains. “But the relationship between X-ray attenuation and proton relative stopping power (RSP) is uncertain. Moreover, experience from human tissue cannot be easily translated to calibration curves for murine tissue. Hence, we decided to rely on proton imaging to provide low-dose pre-treatment alignment, along with the possibility of tomographic imaging for more accurate stopping power information.”
Parodi and colleagues have now performed a Monte Carlo (MC) study of their proposed pCT system, to assess its feasibility and optimize the design of the detector components.
Detector designs
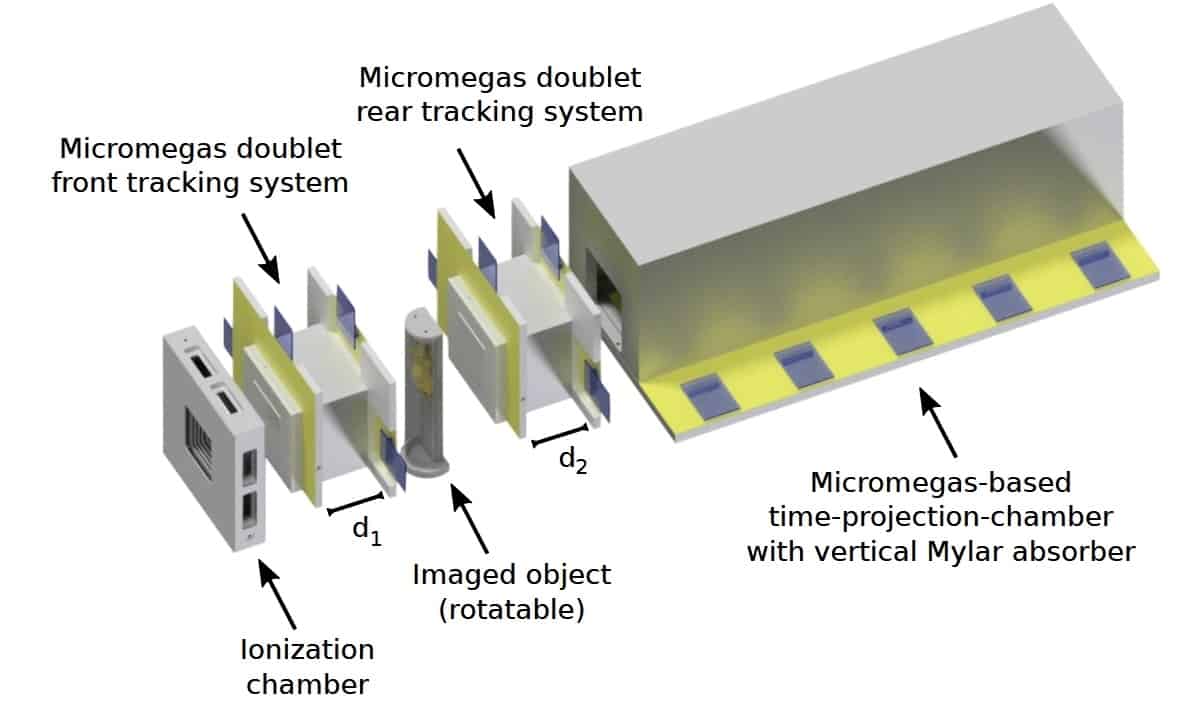
The SIRMIO pCT is a single-particle tracking system, comprising tracking detectors on either side of the imaged object and a residual range detector placed downstream. The tracking detectors, which estimate particle trajectories through the object, each contain a doublet of Micromegas planar gaseous detectors. The residual range detector, which measures the energy lost by each proton (expressed as water-equivalent path length, WEPL), is a Micromegas time-projection-chamber with vertical Mylar absorbers.
“Low-energy proton imaging is challenged by scattering in the detector,” says co-author Jonathan Bortfeldt. “Hence, we chose gas-based Micromegas to realize the lowest possible material budget, while offering high spatial resolution and high count-rate capability. The proposed technology is scalable and could be tailored in future to clinical application.”
The spatial resolution of a reconstructed pCT image is determined by the precision of the estimated proton trajectories. For Micromegas detectors, this depends on two fixed factors: the pitch of the readout strips (500 μm) and the detector-to-object distance (4 cm); plus two adjustable parameters: the material budget and the spacing between planes in each doublet. Using MC simulations of a 75 MeV scanned proton beamline, the researchers aimed to optimize these latter two parameters.
Conventional Micromegas use three layers of copper strips in the readout structure. To reduce the material budget, first author Sebastian Meyer simulated two redesigns: removing the last layer of readout strips from the active area; and replacing the 33 μm copper strips with 9 μm thick aluminium strips. Simulations of the three structures in a water phantom showed that, for the aluminium strip design, the average RMS path estimation deviation was around 0.29 mm, compared with 0.36 and 0.39 for the two- and three-layer copper strip designs, respectively.
The researchers also varied the distance between the two planes in the tracker doublets, from 1 to 10 cm. They found that a spacing of at least 7 cm maximized the accuracy of the estimated proton trajectories, giving an average RMS path deviation of 0.18 mm. To avoid spatial resolution degradation, they chose 10 cm spacing as optimal.
Simulated pCT images of a slanted edge phantom revealed spatial resolutions of 1.9, 2.2 and 2.8 mm–1, for the two- and three-layer copper and aluminium strips designs, respectively. The team notes that this performance is comparable to that of X-ray cone-beam CT systems commonly used in preclinical research.
To optimize the residual range detector, the researchers quantified RSP accuracy for Mylar absorber thicknesses of between 250 and 1000 μm, by simulating pCT of a water phantom with tissue-equivalent inserts. An absorber thickness within 500–750 μm gave the best trade-off between WEPL resolution and detector complexity, providing sub-0.5% RSP accuracy.
Small-animal studies
Using their optimized pCT design – aluminium detector layers spaced by 10 cm and a residual range detector with 500 μm thick absorbers – the researchers simulated pCT images of a mouse head. The pCT images clearly resembled the reference anatomy at a low noise level, but with blurring due to the limited spatial resolution.
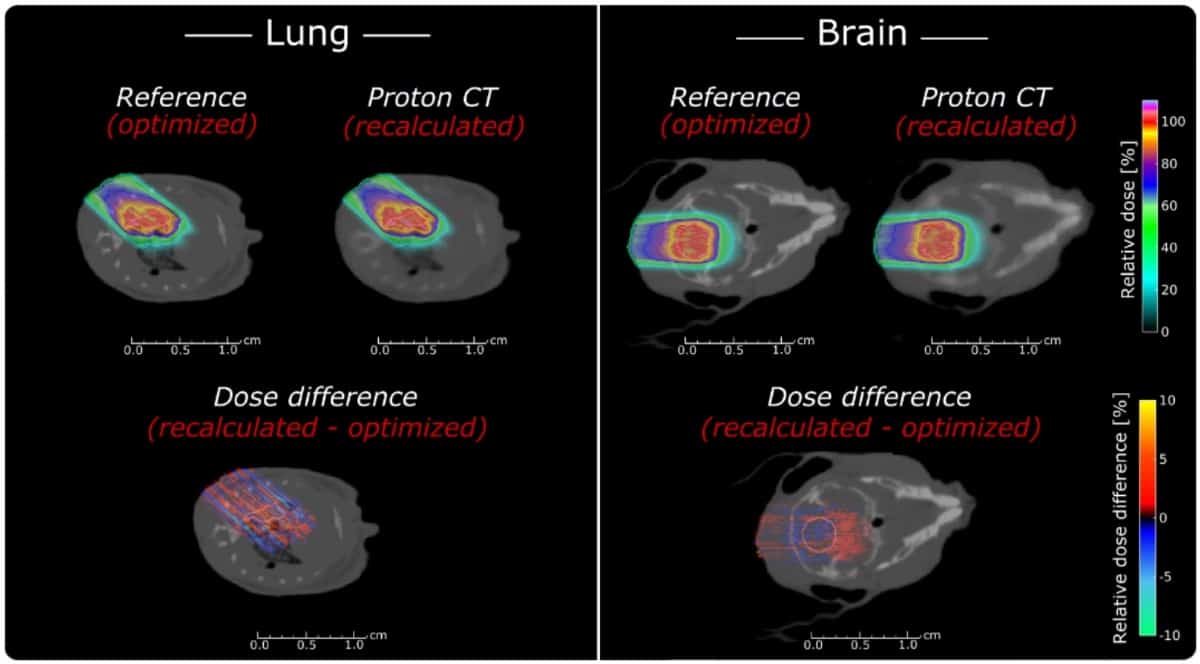
To investigate whether such pCT images are suitable for treatment planning, they used a MC-based proton treatment planning system to plan brain and lung tumour treatments in a mouse model. Comparing optimized reference plans with plans recalculated on the pCT images showed that pCT enabled sub-millimetre accuracy. The average relative range errors were -0.02±1.42% and +0.87±0.98%, for the lung and brain cases, respectively. The corresponding absolute water-equivalent range differences were -0.01±0.20 mm and +0.09±0.10 mm.
Small-animal irradiation platform performs preclinical proton studies
Parodi notes that both detector components of the pCT system are already under construction and being tested. “A full-size tracking Micromegas prototype with aluminium strips, as well as a reduced version of the full-scale time-projection-chamber range telescope with Mylar foils as absorbers, have been already realized in-house and successfully commissioned in low-energy proton beams from the Munich Tandem accelerator,” she explains. “Currently, larger scale prototypes are being realized and the full system pCT realization is expected for fall 2020.”
The study is published in Physics in Medicine & Biology.




