
Proton therapy is becoming an established precision cancer treatment. Studies comparing outcomes of proton and X-ray therapy, however, are mostly retrospective clinical or in vitro cell studies — preclinical investigations of proton therapy are relatively rare. A small-animal irradiation platform that can deliver both photons and protons could enable comparative in vivo research into the biological differences between proton and X-ray beams.
With this aim, researchers at the University of Pennsylvania have coupled an image-guided small-animal X-ray irradiation platform (Xstrahl’s SARRP) to a proton beamline at the Roberts Proton Therapy Center and designed a quality assurance (QA) protocol for the system (Phys. Med. Biol. 10.1088/1361-6560/ab20d9).
“The main difficulty for preclinical studies is access to the proton beam treatment rooms and equipment, which are primarily dedicated for clinical use,” says senior author Eric Diffenderfer. “We chose to move the department’s SARRP to the dedicated proton research beamline, therefore providing both the imaging and alignment functions of the SARRP instrument and access to the system during clinical treatment hours.”
The SARRP was mounted on rails so it could be moved into and out of the path of an experimental proton beamline from the centre’s clinical cyclotron. “This affords easier access to the robotic animal positioning stage, which is useful when the instrument is being used for X-ray studies, and allows us unimpeded access to the proton beamline with the SARRP positioned out of the way,” Diffenderfer explains. It also enables the proton system to employ the SARRP’s on-board cone-beam CT (CBCT) for soft-tissue delineation and alignment.
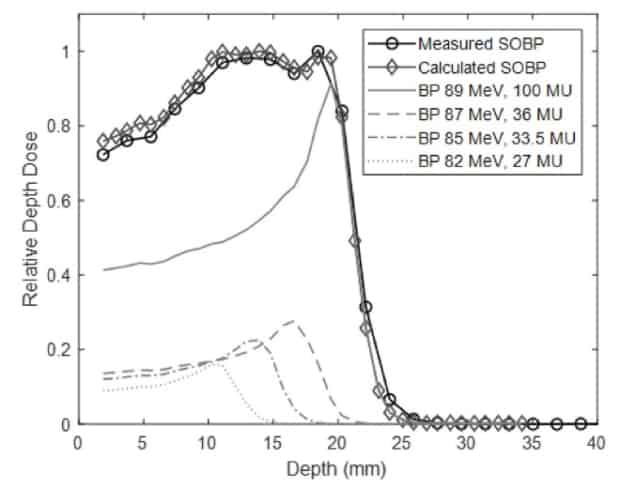
The researchers designed a collimation system to shape the proton field from 0.5 to 10 mm in width. They commissioned the beamline to deliver 77–96 MeV proton beams, with Bragg peak ranges of 4–30 mm in water. For each energy, the distal dose fell to below 10% of its peak within 2 mm. The system could also create spread-out Bragg peaks (SOBPs) with a range of up to 29.5 mm and a width of up to 24 mm.
“We showed that a high-energy clinical proton beam could be degraded and collimated to a range and beam size appropriate for preclinical studies, with minimal degradation of beam characteristics,” says Diffenderfer. “Furthermore, the energy switching capabilities of the clinical system can be easily used to generate arbitrary width SOBPs.”
QA protocol
The researchers developed a dedicated QA protocol based on a 3D printed phantom that holds two Gafchromic films: one to verify proton beam alignment with the SARRP and one to verify depth–dose characteristics. A small high-Z insert affixed to the front face enables CBCT targeting.
To align the SARRP with the proton beam, the researchers placed the QA phantom on the robotic positioning stage and acquired a CBCT. They used the stage to move the SARRP to the centre of the high-Z insert and delivered a proton beam to the front film. The stage was then shifted to align the centre of the proton field with the insert. Finally, to verify the co-linearity of the SARRP isocentre with the proton field, they delivered a second proton beam onto a second film on the phantom.
Performing this QA procedure improved the discrepancy between the SARRP and proton beam isocentres from 2.67±0.38 mm to 0.12±0.04 mm. The researchers note that the QA protocol, which should be performed prior to any small-animal experiments, takes 10–15 minutes and is easy to implement.
Animal irradiation
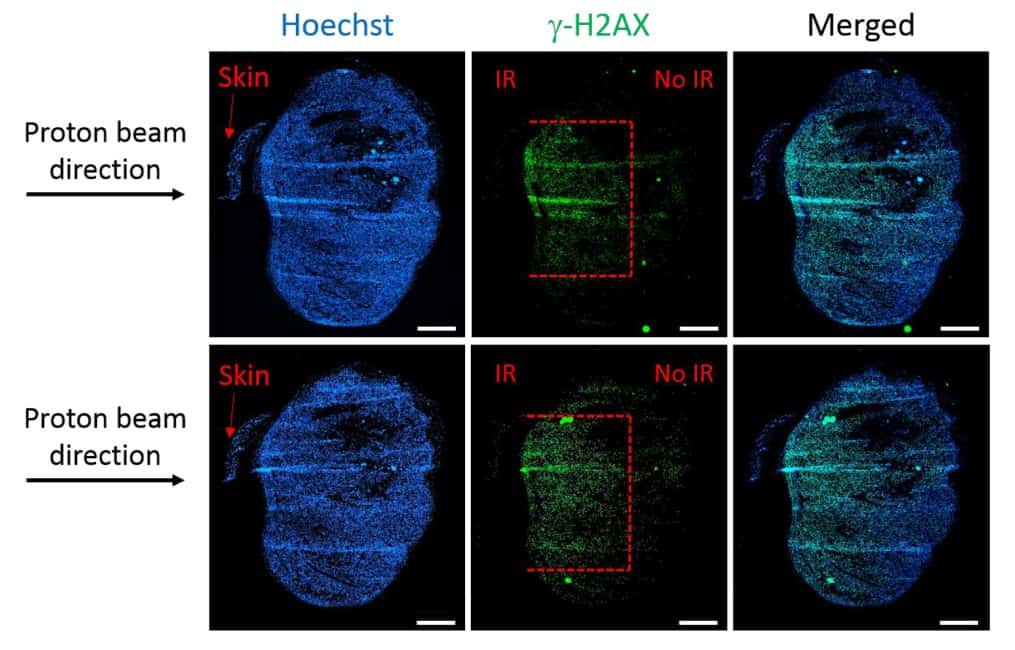
One key objective of this study was to determine whether the set-up could restrict tissue damage to a specified depth. To investigate this, Diffenderfer and colleagues used a 5 × 5 mm collimator to deliver an 89 MeV beam to a flank tumour on a mouse. They placed bolus in front of the target to locate the Bragg peak 3 mm deep into the tumour.
To analyse the post-irradiation damage, they used immunofluorescence staining to detect double-strand breaks. Staining tumours harvested one hour after 4 Gy of proton irradiation showed that the depth of damage was restricted to 3 mm, equivalent to the Bragg peak depth. The width of the damaged area (4–5 mm) corresponded to the size of the collimator, while the remainder of the tissue exhibited no visible DNA damage.
The researchers concluded that this system is the first to deliver a SOBP from a clinical cyclotron combined with a commercial SARRP for X-ray irradiation. They suggest that the coupled proton–SARRP system will become an important tool in proton radiobiological studies and that this study shows how integrated X-ray/proton systems can be developed at clinics, rather than relying on dedicated facilities.
The team has a number of proton versus photon studies planned and underway, in collaboration with the radiation biology group at University of Pennsylvania. “Of particular interest are preclinical studies to investigate FLASH radiation with protons and connections between immunotherapy and radiation,” Diffenderfer tells Physics World.



