Radiotherapy is one of the most common cancer treatments, effectively prolonging survival times and increasing cure rates for cancer patients. However, radiotherapy-induced bone damage – including reduced bone mass, increased bone fragility and higher risk of fractures and osteonecrosis – remains a common problem that currently lacks effective countermeasures.
Radiation causes this damage by supressing the growth, survival and maturation of bone-forming cells called osteoblasts, thus inhibiting bone formation. One potential remedy could be exposure to non-invasive electromagnetic fields (EMFs), which are known to stimulate osteoblast growth and differentiation, and could mitigate the effects of irradiation. Now a research team in China has identified the optimal EMF waveform to maximize the efficacy of such a treatment, reporting the findings in Science Advances.
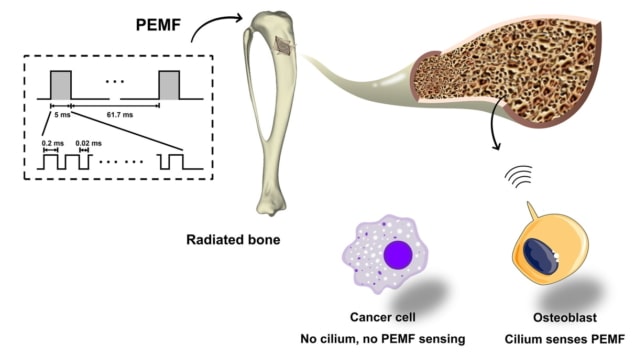
Da Jing, from Fourth Military Medical University, and colleagues first subjected bone cells to EMF stimulation using various waveforms, including sinusoidal EMF, single-pulsed EMF and pulsed-burst EMF (PEMF). To assess the cells’ response, they monitored real-time intracellular calcium ion (Ca2+) signalling, one of the earliest cellular responses to external stimuli.
The team found that PEMF induced more robust intracellular Ca2+ signalling in irradiated osteoblasts than the other waveforms, characterized by unique Ca2+ oscillations with multiple Ca2+ spikes. Further analyses showed that a previously unidentified PEMF waveform with a magnetic field intensity of 2 mT and a frequency of 15 Hz elicited the strongest response in osteoblasts. In contrast, this PEMF waveform had no effect on other types of irradiated bone cell (osteoclasts and osteocytes).
Next, the researchers investigated whether PEMF delivered using these optimal parameters could mitigate radiation-induced bone loss in vivo. In studies on rats, they exposed one hindlimb to two 8 Gy doses of focal radiation (one day apart) and used micro-CT to assess the bone structure 45 days later. The irradiated limbs exhibited significant trabecular bone loss, including a roughly 50% decrease in bone volume fraction and bone mineral density compared with the unirradiated side.

A second group of rats received daily whole-body PEMF (2 hr/day) for the 45 days following irradiation. This treatment restored bone mass and mechanical properties in irradiated hindlimbs to the level of non-irradiated limbs, by rescuing osteoblasts. The team note that PEMF had no effect on the animals’ body weight or food intake.
Having shown that PEMF exposure could mitigate radiation-induced bone loss, it’s also essential that the PEMF does not adversely impact the tumour treatment. With this in mind, the researchers compared the sensitivity of osteoblasts and various tumour cells (breast cancer, colon cancer, malignant melanoma and osteosarcoma cells) to PEMF.
Irradiation reduced cell viability and promoted apoptosis in all of the cell types. Crucially, although PEMF improved osteoblast viability and inhibited osteoblast apoptosis, it had no effect on viability or apoptosis in any of the tumour cells at any time point.
Living bioink could enhance bone repair and regeneration
The researchers attribute this selectivity to the presence of primary cilia – sensory organelles that detect and translate extracellular mechanical cues – that act as PEMF sensors. These primary cilia are highly abundant in osteoblasts, but absent in most tumour cells. In an experiment where the generation of primary cilia in irradiated osteoblasts was blocked, the PEMF-mediated increase in osteoblast survival and differentiation almost completely disappeared.
“Considering that, among all bone cell types, osteoblasts are particularly sensitive to radiation, this PEMF regimen, which induces the specific activation of osteoblasts, seems to be a promising and highly efficient approach against radiation-induced bone damage,” the researchers conclude.