Bacteria can colonize a vast number of surfaces in everyday life, from water pipes to teeth, spreading harmful disease in the process. Scientists had assumed that the growth of such colonies relies on bacteria being able to propel themselves towards sources of food, but a group of physicists in Scotland has now shown that colonies expand using nothing more than the simple mechanical repulsion between bacteria that takes place when they grow and bump into one another. This insight could improve our understanding of antibiotic resistance, say the researchers, and may even help in the fight against cancer.
Scientists use computer models of bacterial colonies to better understand a number of key characteristics of these ubiquitous structures. One parameter of great interest is a colony’s speed of growth because this determines how quickly disease can spread. Another important characteristic is a colony’s shape. Bacteria reproduce rapidly, which increases the possibility that they will mutate and acquire resistance to antibiotics. But reproduction requires nutrition and it is possible that the newly formed bacterium will be beaten by neighbouring cells in the race to reach the nutrients that are more abundant on the edge of the colony. The shape of the colony can dictate the outcome of that race.
According to existing models, which are based on a theory developed by biologist Ronald Fisher and mathematician Andrey Kolmogorov in the 1930s, the growth rate and shape of bacterial colonies depend on both a Brownian-motion-like diffusion of nutrients and a random but active motion on the part of the bacteria. However, these models fail to describe the behaviour of colonies growing on a surface, where bacteria are often unable to propel themselves.
Bacteria as ‘active matter’
In the latest work, Fred Farrell and colleagues at the University of Edinburgh, working with Oskar Hallatschek of the Max Planck Institute for Dynamics and Self-Organization in Göttingen, Germany, set out to establish the importance of mechanical forces in the growth of dense colonies of bacteria on solid substrates. Part of a growing number of physicists investigating “active matter” that exists far from thermal equilibrium, the team was also motivated by recent research showing that mechanical pressure can affect the growth and death rate of cells, including cancer cells.
The researchers model the evolution of non-self-propelling single-celled bacteria, starting with a single cell or a row of cells, which are surrounded by nutrients that they gradually deplete to grow and divide. Each bacterium is considered to be an elastic rod that grows along its length and which splits into two when it reaches a certain size. As it expands, the bacterium pushes against its nearest neighbours, creating movement by virtue of the elastic force between it and them.
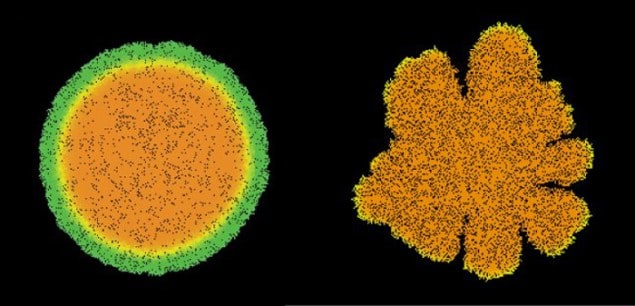
The researchers found that this mechanical force pushes the colony outwards, allowing it to overcome surface friction. An increase in the strength of the pushing force leads to a faster growing colony. They also discovered that the shape the colony takes on as it expands depends on the ratio of the cells’ growth rate to the amount of nutrients available. When nutrition levels are low the colony forms branches to find more food, whereas with bountiful supplies the colony becomes circular, as is observed experimentally.
No diffusion needed
Contrary to the Fisher–Kolmogorov models, this behaviour was achieved without the diffusion of the bacteria and it also relied little on diffusion of the nutrients. Farrell’s colleague Bartek Waclaw points out that the results from the new model could be tested experimentally by confining a bacterial colony to 2D inside a microfluidic array and then imaging it to see how quickly it grows. Whereas the older models predict that the growth should be linear, the new one says it should either be slower than linear or exponential.
Having only two dimensions, however, the model’s utility will be limited, according to Waclaw. Although he adds that a basic 3D extension of the model does reproduce the main results. He explains that newly formed bacterial colonies can exist briefly as a single layer of cells, but colonies quickly build up successive layers. In addition, he points out, many bacteria exchange chemicals to communicate with each other and such signals are not incorporated in the current model. He says, however, that the model could mimic what happens at the early stages of the skin-cancer melanoma, which, he explains, starts out as an essentially flat colony of cells.
Looking at mutations
Waclaw adds that the group is now working on an extended version of the model that allows them to investigate directly how the mechanical properties of bacteria affect the rate of production of potentially antibiotic-resistant mutations. To do so the researchers assume that a certain fraction of the bacteria are mutant varieties and that these cells can grow a little faster than the rest. They then calculate the probability that a drug-resistant mutant cell can reach the nutrients ahead of its rivals and form a critical mass of cells.
A long-term aim of this research, says Waclaw, is to develop drugs that can control the mechanical properties of cells to lower the odds of those cells acquiring antibiotic resistance. “This is just a hypothesis,” he cautions, “but the ultimate hope is that it will one day be possible to modify mechanical interactions by applying a drug.”
The research is published in Physical Review Letters.



