A team of physicists has uncovered two unexpected twists in the tale of how spin-polarized electrons are transported along carbon nanotubes. By trapping just one electron in a tiny length of nanotube, the team found that the motion of the electron has a significant effect on the direction of its spin — and that this effect is very different for “holes”, which are positively charged particles.
Both of these results show that carbon nanotubes (CNTs) — sheets of carbon one atom thick that have been rolled up to create tiny cylinders — are unlikely to be any good at transporting spin-polarized electrons far enough to make them useful in “spintronic devices”, which exploit both the charge and the spin of electrons. However, the new work suggests that CNTs could be better at manipulating electron spin in spintronic devices — or as quantum bits (qubits) in quantum computers.
Spin-orbit coupling
CNTs have a number of desirable electronic properties such as high conductivity, and researchers have already used them to create prototype devices such as diodes and transistors. Many physicists had also believed that CNTs would be very good at transporting spin-polarized electrons, making them ideal for use in spintronic devices and qubits.
Conventional conductors like copper, in contrast, are bad at conducting spin-polarized electrons because of “spin-orbit coupling”, in which the spin of the electron is deflected by an effective magnetic field that is generated by the electron’s orbital motion relative to the material’s atomic nuclei.
But as the strength of this effect is proportional to the fourth power of the number of protons in the nucleus, it should be almost negligible in carbon, which has an atomic number of six, compared to copper with atomic number 29. Carbon nanotubes should therefore be great materials for ferrying spin-polarized electrons around because their spins will not flip very often from up to down, say, as they move. Indeed, it has already been shown that spin-polarized electrons can travel hundred of micrometres in silicon, which also has a low atomic number of 14.
This view had been supported by several independent studies of electrons in CNT “quantum dots”, tiny lengths of nanotube containing just handfuls of electrons. While some deviations from the expected behaviour was seen, these were explained in terms of interactions between the electrons, not spin-orbit coupling.
One electron per dot
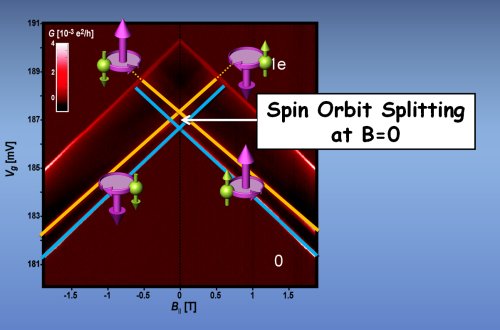
Now, Shahal Ilani, Ferdinand Kuemmeth and colleagues at Cornell University in the US have shown that the spins are in fact strongly coupled to the orbital motions of electrons in CNT, by using a nanotube quantum dot containing just one electron (Nature 452 448).
Their quantum dot was a length of CNT about 500 nm long which had metal electrodes at each end (the source and drain). Two gate electrodes were placed under the CNT, one at the source end and one at the gate end. By adjusting voltages applied to the two gates, the team were able to trap a single electron within a 200 nm portion of the CNT.
The energy levels of the electron were then measured by scanning a second voltage between the source and drain. At certain voltages the electron will tunnel in and out of the dot and these voltages can be used to determine the energy levels of the electron in the CNT.
A single electron in a CNT is expected to have four possible combinations of spin up/down and clockwise and counter-clockwise orbital motion around the tube’s circumference. If there was no spin-orbit coupling, all four states should have the exact same energy.
Instead, the team discovered that the energies of these states were split in two according to the relative orientations of the spin and the orbital motion of the electron around the tube. The higher energy level corresponded to the spin and orbital magnetic moments pointing in opposite directions and the lower level to spin and orbital moments in the same direction. The energy gap between the two levels is about 0.37 meV — which is several orders of magnitude greater than expected in a flat sheet of carbon.
Four-way splitting
The team then repeated the measurement while applying an external magnetic field along the length of the CNT. This further split the energy levels into four, corresponding to the relative orientations of the spin and orbital magnetic moments to the applied field — exactly as expected from strong spin-orbit coupling.
While this unexpectedly strong interaction means that free spins might not be the best way to realize quantum bits in CNTs, Ilani believes that if properly exploited, this coupling could yield new types of robust quantum bits in which the spin and orbital degrees of freedom are entangled. He further notes that this coupling adds a new tool that was so far missing in carbon based system — the ability to control the spin state of an electron by intentionally manipulating its orbits.
Holes versus electrons
According to Kuemmeth, the experiment yielded another unexpected result — that spin-orbit coupling affects electrons and holes differently in CNTs. In semiconductor physics, a “hole” is the absence of an electron, which behaves like a positively charged particle. In most semiconductors, holes have different physical properties than electrons, but physicists had thought in CNTs, apart from opposite charge, holes and electrons in NTs should have the exact same physical properties.
However, by measuring the energy states of a single hole in a the same CNT dot, the Cornell team discovered that the spin-orbit interaction causes anti-parallel alignment of spin and orbital moments for a hole, the exact opposite than it does for an electron.



