A new way to extend the lifetimes of charge carriers in solar cells has been unveiled by researchers in Spain. The technique involves creating an aggregate of two different kinds of quantum dots, which can be made using low-cost solution-processing techniques. According to the researchers, the method could be used to boost the performance of solar cells – even those based on photovoltaic materials that have relatively poor optoelectronic properties.
Solution-processed inorganic solar cells are made by depositing layers of quantum dots – tiny pieces of semiconductor – in colloidal suspension. The devices have shown much promise because they can absorb light over a wide spectrum of wavelengths. This is a result of the fact that the electronic band gaps in a quantum dot can be tuned over a large energy range by simply changing the size of the dot. They are also comparatively cheap to produce.
However, only a limited number of materials have been exploited in this type of solar cell. When light is absorbed by a solar cell it liberates pairs of charge carriers (electrons and holes), which must endure for a long enough time to travel through the device to where they can become a useful electrical current. The problem is that only a handful of materials – two common examples being lead- or cadmium-based quantum dots – have carrier lifetimes that are long enough.
Avoiding toxic elements
“However, lead- and cadmium-based quantum dots are based on toxic elements, so we researchers are actively looking for other, safer materials, even if their optoelectronic properties are poorer – but then we need a device structure to accommodate them in a useful way,” explains Gerasimos Konstantatos of the Institut de Ciències Fotoniques in Barcelona, who led this latest research.
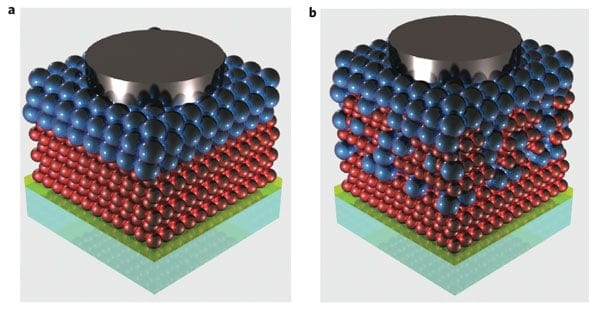
Konstantatos’s team created a “bulk nano-heterojunction” in a solar-cell device consisting of p-type and n-type semiconductors. The two materials were mixed in such a way that, when exposed to sunlight, photogenerated electron-hole pairs were then able to separate at the nanoscale and travel along the device via two very different paths, something that reduced the chances of them recombining.
The device consisted of a nanocomposite comprising a mixture of p-type PbS quantum dots and n-type Bi2Si3 quantum dots (see figure). This mixture is sandwiched between a layer of pure Bi2Si3 quantum dots – which transports electrons and blocks holes – and a layer of PbS quantum dots, which has the opposite transport properties. To determine the relative efficacy of the mixture layer, the team also made “bilayer devices” with an abrupt junction between the two types of quantum dots. Konstantatos and colleagues found that the power-conversion efficiency of the bulk nano-heterojunction devices was found to be around 4.8%, a value that is three times higher than the bilayer devices with sharp junctions.
Longer lifetimes
To work out the reason for this improved efficiency, team member Arup Rath and colleagues set about measuring the lifetimes of charge carriers in the devices while exposing the cells to varying optical intensities. Although both devices show long lifetimes at low light intensity, at higher intensities similar to sunlight, the device contains carriers with shorter lifetimes because electron and holes combine at a faster rate here. Carriers in the bulk nano-heterojunction device, on the other hand, appear to last three times longer than in the bilayer structure since electrons and holes recombine at a significantly slower rate.
“Although the power-conversion efficiency of our cells is still a bit lower than record efficiency devices based on PbS quantum dots and titania n-type electrodes, it does demonstrate the proof-of-principle,” Konstantatos says. “What is more, unlike previous studies that relied on either sputtered oxide-electron acceptors or high-temperature sintering at 500 °C, our technique works using a fully solution-based process and at low temperatures of less than 100 °C – non-negligible advantages for low-cost roll-to-roll manufacturing, for example.”
The results are described in Nature Photonics.