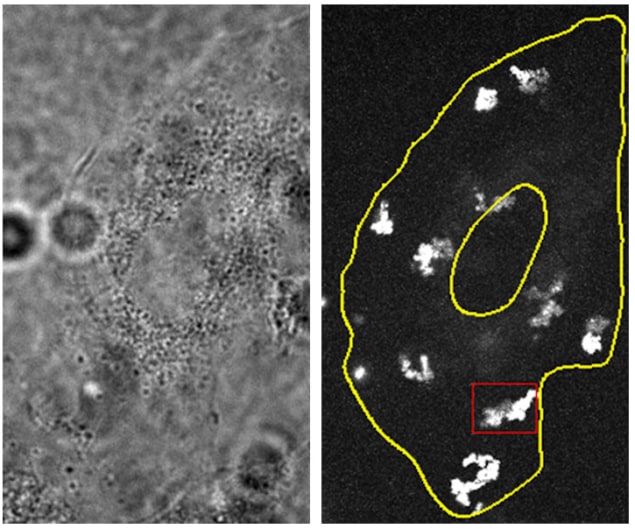
Quantum dots diffuse within living cells in a nearly two-dimensional fashion. This result, which was obtained using a new 3D microscopy technique that can track single particles, sheds fresh light on intracellular diffusion – a process that is critical for moving molecules around the cell and for mediating other important activities. According to study leader Hui Li, a biophysicist at the Chinese Academy of Sciences in Beijing and Beijing Normal University, the 2D motion he and his colleagues observed is robust and stems from the complex architectures of the flat “adherent” biological cells they studied.
Quantum dots make ideal probes for studying intracellular diffusion in living cells. They are similar in size to intracellular macromolecules and can be made to mimic biological materials relatively easily, by coating their surfaces with organic molecules. Previous studies, however, relied mainly on two-dimensional measurements of their movement, with the assumption that three-dimensional diffusion is an extension of 2D diffusion and is isotropic.
The new work reveals that diffusion is in fact highly anisotropic, thanks to the heterogenous architectures of various cell structures. From the quasi-2D diffusion behaviour they observed, Li and colleagues infer the presence of planar structures within the cytoplasm – the thick solution composed mainly of water, salt and proteins that fills each cell and is enclosed by the cell membrane. They also suggest that these planar structures provide a means of rapidly and efficiently transporting molecules by diffusion within the cells.
3D single-particle tracking microscopy
Li and colleagues obtained their result using an extension of a 2D single-particle tracking (SPT) instrument that they developed in 2015. Like its predecessor, the new 3D SPT relies on tracking single quantum dots in “adherent” cells – one of the most common types of biological cell, and the model of choice for cellular studies.
While the earlier method was only able to measure the positions of particles in the lateral (x and y directions), it nevertheless revealed a heterogenous and compartmentalized diffusion behaviour in the endoplasmic reticulum – a cellular structure that plays a major role in protein synthesis, folding and transport. It could not, however, show how the dots actually diffused through the cytoplasm in all three directions in space. The new method overcomes this shortcoming because it can measure the axial (z-direction) positions of the particles as well as their lateral ones – with a resolution as small as 35 nm.
Joining up the dots
To improve on their 2D SPT microscope, the Beijing researchers had to construct two additional components for it: a focus-locking apparatus to eliminate vertical drift; and a two-focal imaging apparatus to measure axial positions of particles from off-focused diffraction rings.
Nanotweezers probe single cells
In their experiments, the researchers loaded their quantum dots into the cytoplasm of cultured human (A549) cancer cells. Once they localized the particles using their new 3D SPT approach, they then “joined up” the dots to construct their trajectory through the cytoplasm. This technique allowed them to analyse trajectories in terms of motion type (that is, Browning motion, sub-diffusion or confined motion), and also yielded information on parameters such as diffusion rates.
“Intracellular diffusion is critical for molecule translocation in cells and mediates many important cellular processes,” Li tells Physics World. “Our finding suggests that cells may utilize the architecture of the cytoplasm to control the intracellular diffusion dynamics and regulate macromolecule transport.” Indeed, the quasi-2D diffusion of the dots with constrained movement in the axial direction appears to effectively promote molecular translocation and shorten particle diffusion times.
Members of the Beijing team, who report their work in Chinese Physical Letters, now plan to combine their 3D SPT technique with dynamical subcellular imaging to better explore the relationship between the quasi-2D diffusion they have observed and cell architecture. “We are also investigating intracellular diffusion in fast-migrating cells to further elucidate the physical mechanisms behind cell diffusion,” Li says. “This is our most exciting project right now.”




