
A new variation on an inkjet printer can create multi-component tissue scaffolds with features comparable in size to a single cell. Researchers in Germany modified a commercial bioprinter to output droplets of two different polymeric precursor materials simultaneously. When the droplets combine on a surface, rapid crosslinking reactions result in a hydrogel that retains its shape, making it able to form intricate 3D shapes. By altering the composition of the precursors, the mechanical and biological properties of the scaffolds can be made to vary in space, producing complex tissue-like structures that can be used as experimental models or for tissue regeneration (Biofabrication 10.1088/1758-5090/ab2aa1).
3D printing with biological materials has enormous potential as a way to fabricate tissues and even whole organs for medical and research purposes. Typically, polymeric precursors and suspensions of living cells are deposited as liquid droplets, forming stiff hydrogels only when heat or ultraviolet radiation are applied to induce crosslinking between the component molecules. This makes the method unsuitable for forming structures with very fine detail, as the droplets tend to spread out before the gelation process is complete.
To tackle this limitation, Ralf Zimmermann, at the Leibniz Institute of Polymer Research Dresden (IPF), and colleagues at IPF, Dresden Technical University and GeSiM, developed an inkjet bioprinter with two separate piezoelectrically controlled print nozzles. The researchers used one nozzle to dispense sub-nanolitre droplets of a solution containing the star-shaped polymer polyethylene glycol (starPEG) that had been functionalized with thiol, and the other to dispense a precursor functionalized with maleimide. When droplets from each nozzle converge on the substrate, the two functional groups react almost immediately, crosslinking to form a hydrogel before the mixture spreads.
Printing with a solution of starPEG in each nozzle can produce hydrogel structures with a resolution of 50 μm — a significant improvement on what has been achieved with similar gel systems until now. But while this represents a significant technical accomplishment, applications for such single-component structures are limited. When biological cells populate a scaffold, their behaviour – how they propagate and differentiate, and the tissues and structures that they form – depends on the biochemical and physical cues in their environment. This means that effective tissue scaffolds need to contain regions that vary in composition and mechanical properties so that cells are prompted to develop in a specific way.
Zimmermann and colleagues achieved this by replacing the maleimide-functionalized starPEG with heparin, which alters the mechanical strength of the hydrogel. The researchers found that different ratios of starPEG to heparin produce a material with a Young’s modulus that ranges from 2 to 28 kPa. Being able to fine-tune this property of a scaffold is important, because cells feel and respond to the stiffness of their substrate: mechanical forces are one of the factors that influence the outcome of stem-cell differentiation.
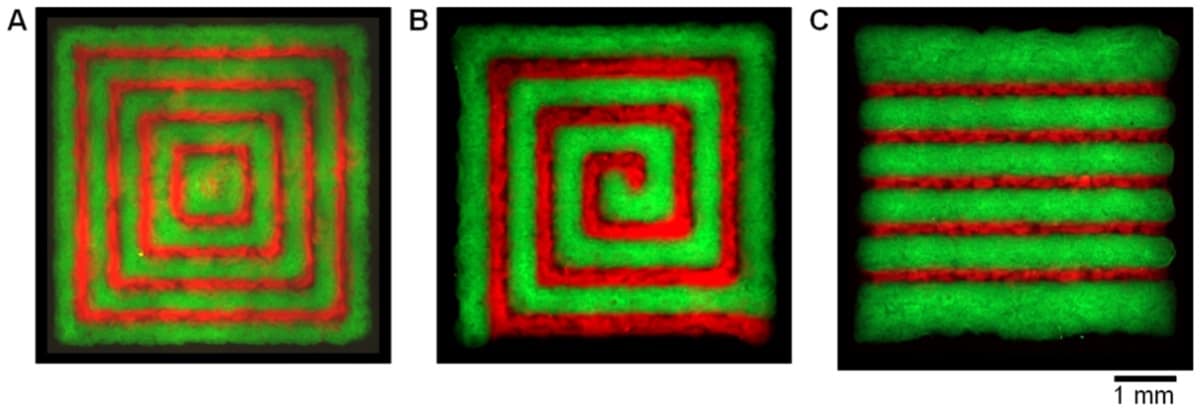
Adding heparin to the mixture also allowed the researchers to incorporate into the scaffold other biologically active molecules, for which the heparin acts as a vehicle. These biomolecules promote certain desired cell behaviours: the RGD peptide, for example, improves cellular adhesion with the scaffold, while a platelet-derived growth factor encourages cells to migrate.

The past, present and future of 3D bioprinting
Having fine control over how such substances are arranged in a scaffold opens up new possibilities in tissue engineering and regenerative medicine. “The approach can be used to form spatio-temporal morphogen gradients in in vitro experiments, to mimic tissue boundaries in cell-laden hydrogels (to study the molecular transport of drugs across tissue boundaries, for example) or to mimic hierarchical tissue structures (as in implants for regeneration of articular cartilage defects),” says Zimmermann.



