Researchers at North Carolina State University have developed a mathematical model for computing radiation treatments that could substantially reduce side effects, while delivering the same results as conventional radiotherapy (Phys. Med. Biol. 63 015036).
Most radiotherapy treatments are fractionated, with patients receiving the total radiation dose split into multiple treatments delivered over several days or weeks. Such fractionated regimes reduce radiation-induced cell damage, because if the same physical dose is delivered in multiple fractions, it allows healthy cells to recover between treatments. Current clinical protocols stipulate that patients receive the same dose in each treatment session; but this regime may not always be optimal.
“Different doses, carefully planned to minimize side effects, can be just as effective,” explained Dávid Papp, assistant professor of mathematics at NC State University. “However, the extent of this benefit has never been assessed. The algorithms we use now to determine the best personalized treatments don’t work when computing treatments with different dose distributions in different fractions.”
Papp developed and tested a “spatiotemporal fractionation” approach, in which different dose distributions are delivered in different fractions. By hypofractionating parts of the tumour while delivering approximately uniform doses to the surrounding tissue, such treatments can reduce the radiation dose to healthy tissue while maintaining effectiveness against the tumour.
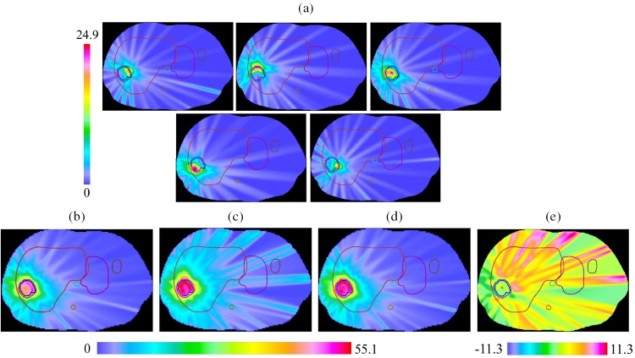
In a proof-of-concept study, Papp and colleagues tested the plan against model slices of five different liver tumours, each representing a unique tumour size or location, to allow comparisons with actual clinical treatments.
“We wanted to see what the quantitative benefits of such a new protocol would be,” said Papp. “How much can you reduce the radiation’s effect on the liver while making sure that the tumour receives a consistent and effective dose? A reduction of 20% would reduce side effects enough to warrant a change in everyday clinical practice.”
The computed spatiotemporal plans reduced the liver dose by 13 to 35%, without compromising other clinical goals. Papp has now begun work on refining the model to make it more robust, with a view toward in vivo testing.
“Conventional radiation treatments don’t necessarily achieve maximum benefit,” Papp says. “Our protocol, by delivering a high single-fraction dose to parts of the tumour during each fraction and a consistent lower dose to the liver and other healthy tissue, could reduce patient side effects substantially while maintaining the same effectiveness as conventional treatments.”



