
Non-oxide ceramics have many potential industrial applications due to their properties at high temperatures, including excellent corrosion resistance, stiffness and light weight. However, the synthesis of such materials, in particular MAX phase ceramics (where M stands for early transition metals, A stands for group 13 or 14 elements and X stands for either carbon or nitrogen atoms), is very costly. It requires processing temperatures above 1,000°C as well as extensive milling to create fine, workable powders from the large porous blocks that common solid-state synthesis methods produce. Furthermore, inert atmospheres or even vacuum are required during these high-temperature processes as the ceramics are subject to oxidation, increasing the cost of processing as well as the necessary equipment, and further limiting their use at an industrial scale. Now, however, researchers at Forschungszentrum Jülich GmbH in Germany led by doctoral student Apurv Dash, have developed a lower-temperature technique dubbed the ‘molten salt shielded synthesis/sintering’ process (MS3 process) which allows processing of high-purity MAX ceramics in air. The process may give this promising class of materials an environmentally friendly path to large-scale use in industry.
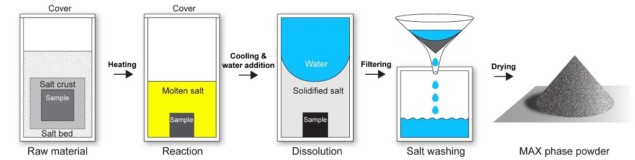
A briny synthesis process
The MS3 process involves encapsulating powdered elemental precursors for the ceramic or metal of interest within a salt matrix (potassium bromide, KBr). In this case the researchers used the metal titanium and the ceramic Ti3SiC2 as model cases. Although salt synthesis is no stranger to ceramics processing, previously non-oxide ceramics still needed an inert atmosphere to prevent oxidation. By using KBr, Dash and his colleagues could exploit its mechanical properties, which make it very ductile at room-temperature, and allow them to compress it to 95% its relative density by coldpressing, so that it forms a gas-tight seal around the sample and shields it from oxidation by the atmosphere. They then heat the salt and sample to high-temperatures to melt the salt, creating a molten salt bath, sintering the metal or synthesizing a new ceramic phase from the elemental precursors in the process.
To remove the salt the researchers then simply dissolve it in water, leaving a highly pure metal or ceramic sample behind. They could then recover the salt from the saline water to re-use it for further processing. The researchers estimate that just 3 l of water would be necessary to generate 1 kg of MAX phase ceramic.
The MS3 process gives great control over the properties of the sample of interest. It can make dense and porous metal samples, as well as fine ceramic powders, reducing the number of expensive and energy-intensive steps required in traditional processing methods.
Mantis shrimp strikes again to inspire tougher composite materials
Furthermore, MS3 allows a reduction in processing temperatures by approximately 100 °C compared with other common techniques. This technique could launch a class of promising materials into large-scale industrial use in a cheap, scalable and environmentally friendly way, potentially creating many applications in the aerospace and biomedical implant industries.
Full details of the research are reported in Nature Materials.



