
MRI-guided radiotherapy systems offer the ability to visualize tumour targets and surrounding organs with high accuracy, with the potential to perform real-time treatment adaptation based on anatomical changes. And for moving tumours, imaging during treatment can ensure that the radiation beam remains focused on the target to maximize healthy tissue sparing.
To mitigate the impact of respiratory motion, for example, one option is to perform gating. The Elekta Unity MR-linac is equipped with an automatic gating functionality in which a comprehensive motion management (CMM) system continuously monitors the 3D tumour motion. Radiation is only delivered when the target lies within a specified gating envelope – when it moves outside of this region, irradiation is paused.
“Although this is highly effective, it means that treatment time is drastically increased,” said Prescilla Uijtewaal from the University Medical Center Utrecht. “Therefore we would like to do multileaf collimator [MLC] tracking instead of gating.”
Speaking at the recent ESTRO 2023 congress in Vienna, she described the new MR-linac tracking approach. The idea is to use the position of the tumour measured by the CMM not to gate the beam, but to move it towards the tumour location using MLC tracking (in which the collimator leaves are shifted to match each new position).
To validate this workflow, Uijtewaal and colleagues developed a dosimetric insert jointly with Medscint and ModusQA that contains gafchromic film plus an array of eight plastic scintillation detectors positioned at the centre and edges of the target. Film dosimetry is low cost and can create 2D dose maps, while scintillators enable instantaneous dose read-out and time-resolved dosimetry. “Combining the two in a single insert provides great dosimetric analysis,” Uijtewaal pointed out.
The insert is designed to fit inside the MR-compatible Quasar MRI4D phantom, which can be programmed to move with patient-derived respiratory motion patterns. The researchers created an intensity-modulated radiotherapy (IMRT) plan for delivery to a 3 cm spherical target in the phantom. They used the dosimetric insert to compare the planned versus the measured dose, for static delivery, motion with MLC-tracking and motion with no-tracking scenarios.
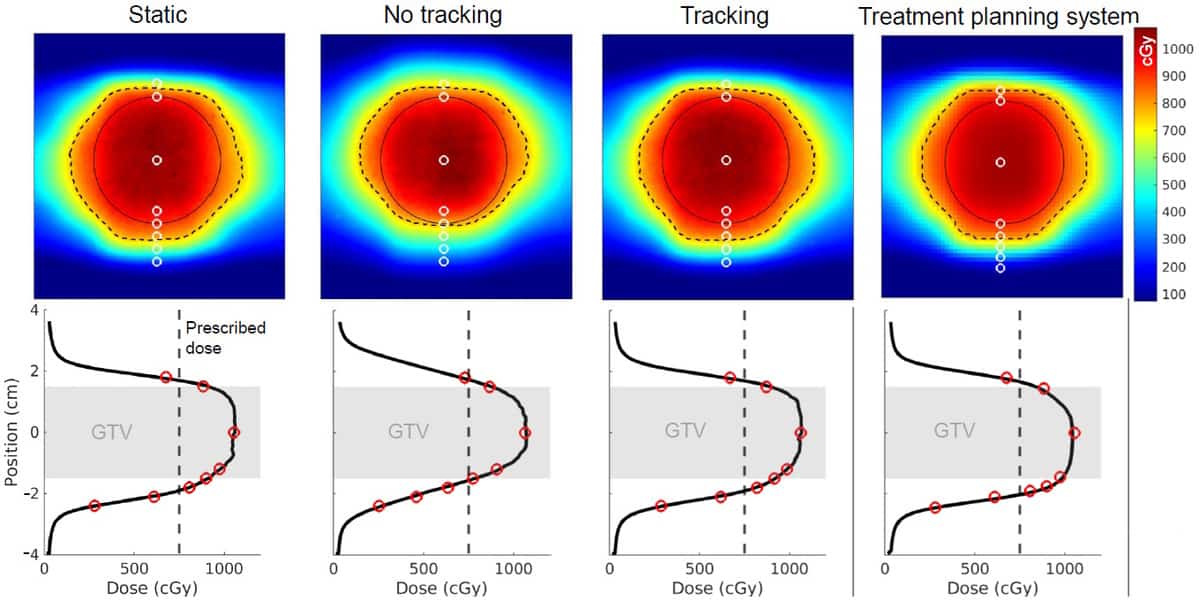
Film measurements revealed that dose maps from plans delivered without tracking differed significantly from the static dose map, with large hot and cold spots at the edges of the target. Applying MLC tracking based on the CCM’s motion vector restored the dose map back to that seen with static delivery, with extremely small differences between the delivered and the planned dose.
The dose measured by each scintillator matched well with the value at the corresponding location on the 2D dose maps. The researchers also used the time-response component of the scintillators to analyse the dose over 400 s with 15 Hz temporal resolution. They assessed the dose measured by all eight scintillators, again in static, tracking and no-tracking scenarios.
The MR-linac: initial clinical experience
“We saw that the tracking dose followed the static dose really well, which means that the tracking was really effective,” said Uijtewaal. “We also saw that both the static and the tracking dose agreed well with the treatment planning system.” Without tracking, measurements deviated significantly from the planned dose, particularly for scintillators located at the caudal edge of the target.
Uijtewaal concluded that “the MR-linac’s comprehensive motion manager is compatible with MLC tracking, facilitating a pre-clinical MLC tracking workflow”. Next, she said, her research group (headed by Martin Fast) plans to enhance the tracking workflow to work towards clinical implementation of MLC tracking on the MR-linac. The team also hopes to increase the number of scintillation detectors, to increase coverage and ultimately implement 3D dosimetry.




