An international team of researchers has developed a new high-resolution x-ray Compton scattering method to visualize the perturbation of redox orbitals in energy-storage materials. The new spectroscopic descriptor would allow the creation of high-performance batteries used in smartphones, laptops and electric cars.
The scientists used high-resolution x-ray Compton scattering profiling to image the redox orbitals since it can provide precise momentum space images. Measuring Compton profiles with Li content maps the evolution of reduction-oxidation (redox) orbitals involved in lithiation/delithiation of battery materials. The novel method helps unravel the mechanism of redox reactions that drive batteries, opening a spectroscopic gateway for improving the material’s performance.
Preparing the orbital camera: Compton profiling of FePO4
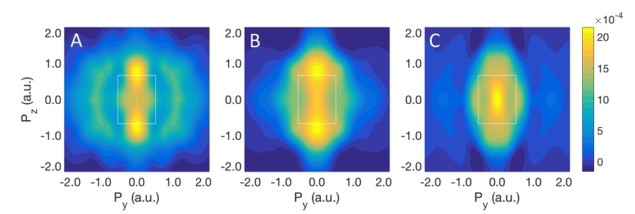
Compton profiles are related to a 2D integral of the electron momentum density (EMD). These are obtained by measuring Compton scattering spectra along different directions of the x-ray scattering vector, and have the same symmetry as the charge density.
The researchers chose lithium iron phosphate (LFP), an olivine family member, as a model material to perform the spectroscopic measurements due its potential as a high-performance cathode material and its intricate delithiation process. This is present as two phases: fully lithiated, LFP, and the delithiated, FePO4.
The experimental Compton profile difference (ΔJ) of the delithiated compound (FePO4) was compared with theoretical Compton profiles. Theoretical profiles were calculated based on three different models of the delithiation process: rigid band model (same as LFP), rigid FeO6 octahedral and relaxed FeO6octahedral.
The experimental ΔJ matched the relaxed FeO6 model, reflecting the oxidation of the Fe2+ to Fe3+ and modification of the Fe-O bond. Such distortion can be highlighted by subtracting the Compton profiles of the relaxed octahedron from the rigid, resulting in a distortion profile, D(p).
“Taking pictures” of redox orbitals
By theoretically calculating the 2D EMD differences of LFP and FePO4, the researchers mapped the modification of the 3D orbitals due to the distortion of the Fe-O bond in the three different models considered. The momentum maps of the redox orbital show how the perturbation of the short-range structure of the materials can produce a localization of the states in different regions of the momentum space. The relaxed octahedral model 2D EMD exhibits the most localization at low momenta, highlighting the importance of the octahedral relaxation on delithiation.
Evaluation of the potential shift
The distortion profile, D(p), also provides information about the loss of the redox potential or potential shift, ΔV, caused by structural perturbation. These distortion profiles, D(p), can be used as descriptors to evaluate voltage shifts so that the energy density of cathodes can be enhanced. The relaxed octahedral model exhibits a potential shift ∆V = –0.62V compared with the rigid model.
Distortion profiles were also derived for Mn, Co and Ni substituted LFPs. Mn introduction increases this potential shift, whereas Co and Ni can reduce it. The results suggest that the strain and octahedral distortions caused by Fe substitution can improve the energy density of pure LFP.
Compton scattering: decoding the mechanism of potential shift
The work was carried out by an international team of researchers from the United States, Japan, Belgium and Poland. The effort was led by Arun Bansil, University Distinguished Professor of Physics at Northeastern University (Boston) with team members Hasnain Hafiz and Bernardo Barbiellini. Compton scattering experiments were performed at the SPring-8 synchrotron light source in Japan led by Yoshiharu Sakurai (JASRRI) with team members Kosuke Suzuki, Yuki Orikasa, Masayoshi Itou, Kentaro Yamamoto, Ryota Yamada, Yoshiharu Uchimoto and Hiroshi Sakurai. Other scientists involved were Vincent Callewaert (Belgium) and Staszek Kaprzyk (Poland). The study was published in the August 23 issue of the journal Science Advances.
The scientists have developed comprehensive theoretical and experimental spectroscopic descriptors that provide a way to decipher the mechanism of lithiation/delithiation and potential shifts in batteries. Compton and distortion profiles offer a quantitative method to observe the changes in momentum space caused by modification of the crystal structure between electron orbitals of transition metals and oxygen.
The newly developed spectroscopic approach allows us to gain a molecular-level understanding of the relationship between structural distortion, potential shifts and metal substitution on working battery materials. Thus, with this novel method, the group of researchers has tremendously contributed to the material science community with one of the many tools needed for deriving the structural-activity relationships of working battery materials to enhance their performance. Their work proves that data derived from the interaction of photons with materials and clever mathematical ways to interpret and process such data can lead us to see what was thought to be blind to the human eye: electronic orbitals.
More information can be found in Science Advances.