Materials that controllably change shape over time – often called four-dimensional (4D) materials – are excellent candidates for advanced tissue engineering applications. Many 4D materials, however, have only been loaded with low concentrations of cells, potentially limiting their use in regenerative medicine.
To address this problem, researchers from the University of Illinois at Chicago have developed a 4D biomaterial system using two types of biocompatible hydrogels. Manufactured as sheets, the materials curl into tubes when exposed to water. Each new hydrogel can support cell densities as high as 100 million cells/mL – approaching the same order of magnitude as that found in developing and healing tissues.
“Using a high density of cells can be advantageous in tissue engineering as this enables increased cell–cell interactions that can promote tissue development,” explains lead author Eben Alsberg in a press statement. The researchers describe their work in Advanced Functional Materials.
Water-activated shape change
Tissue development is a highly dynamic process. Clusters of cells are organized through a series of complex architectural changes until the final tissue structure is formed. It may therefore be beneficial for tissue engineered scaffolds to respond to, and even replicate, the geometric changes that occur within the tissue on the same time scale.
This is where shape-shifting materials shine. The researchers hypothesized that stacking hydrogels with different swelling rates would create a 4D material that gradually changes shape as it absorbs water. By controlling the spatial distribution of each hydrogel throughout the scaffold, the extent of swelling (and therefore shape change) could then be regulated over time.
First, the team investigated the swelling properties of two biocompatible hydrogels: oxidized and methacrylated alginates (OMAs) and methacrylated gelatin (GelMA). They not only found that OMAs swell more than GelMA, but that OMA expansion could be further augmented by altering its chemistry. The higher the degree of OMA oxidation, the faster its degradation rate and the more it expands.
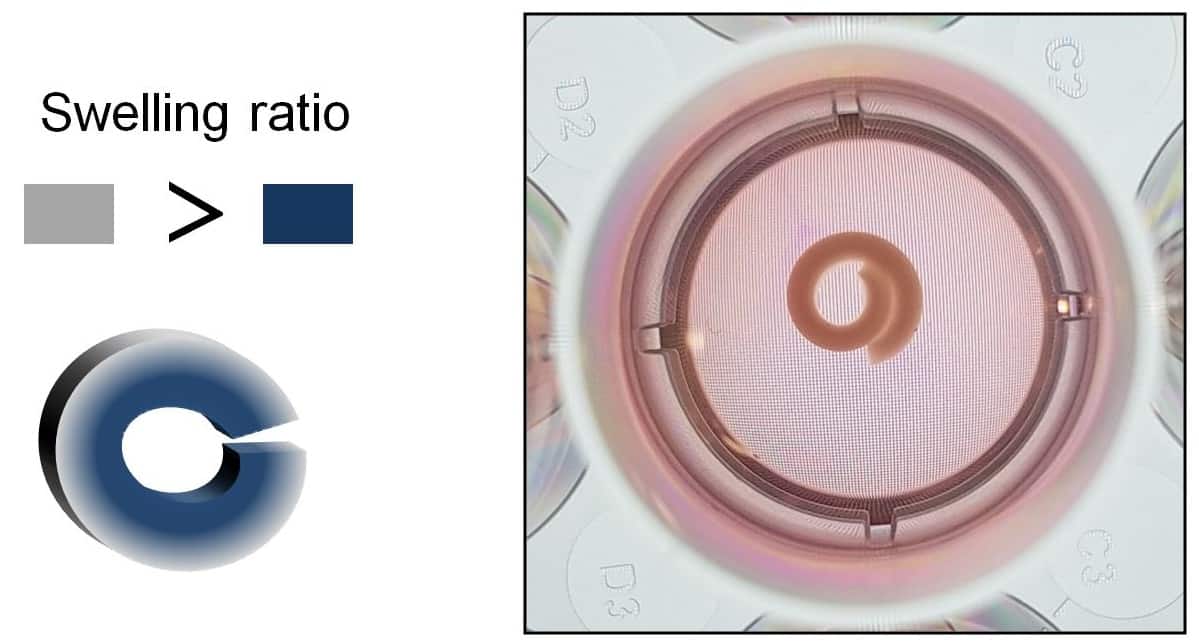
Next, the researchers submerged a series of flat, bilayered OMA/GelMA scaffolds in cell culture media and monitored their subsequent deformation. Over the course of 21 days, each scaffold curved into a “C” shape, with some forming closed circles or even rolled, spiral-like structures. In each case, the OMA layer dictated the overall shape change because it absorbed water the fastest. What’s more, the extent of curling could be controlled by changing the thickness of the OMA layer or varying the OMA oxidation level.
The material is also compatible with techniques like photolithography and bioprinting, which allowed the team to create 4D hydrogels with more complex starting geometries and shape transformations.
“Using our bilayer hydrogels, we can control how much bending the material undergoes and its temporal progression,” says first author Yu Bin Lee.

Record-breaking cell encapsulation
To test the impact of cell density on 4D shape change, the researchers loaded each hydrogel layer with varying concentrations of fibroblast cells or stem cells. The material maintained its rolled structure after three weeks, even at extremely high cell densities (1.0 × 108 cells/mL).
Prior to this study, the highest reported concentration of cells encapsulated within a shape-changing material was 1.0 × 107 cells/mL – 10 times lower than that achieved by the team in this work. Both cell types remained viable throughout the 21-day period. Importantly, the stem cells could perform normal cellular activities like differentiation.
The researchers hope that the material could be used to mimic a range of target tissues that have varied cell concentrations. “This system holds promise for tissue engineering, but may also be used to study the biological processes involved in early development,” says Lee.



