Quality-assurance specialist CIRS has teamed up with GE Healthcare to commercialize an easy-to-use breast-equivalent phantom for contrast-enhanced spectral mammography
For women who undergo breast-cancer screening, the dread news of a suspicious finding on their mammogram or ultrasound scan is all too often compounded by an anxious wait for a follow-up exam that can take weeks to schedule. To streamline that disjointed patient journey and fast-track diagnosis, GE Healthcare is working with clinical partners and technology vendors to enhance the accuracy of 3D mammography – also known as digital breast tomosynthesis (DBT) – by exploiting a complementary imaging modality called contrast-enhanced spectral mammography (CESM).
A case study in this regard is the One-Stop Clinic at the Gustave Roussy Cancer Centre in France. In collaboration with GE, the Paris-based institute is rewriting the rulebook on breast-cancer screening. The goal: to give patients a definitive cancer diagnosis and treatment plan on the same day via multimodality investigations that dovetail the capabilities of GE Healthcare’s Senographe Pristina™ system for DBT imaging and CESM (the latter is known commercially as SenoBright™ HD). To date, more than 20,000 women have participated in the One-Stop Clinic, with 75% of those patients receiving a diagnosis on the same day.
Within this one-stop patient model – and indeed the wider clinical context – CESM has emerged as a key imaging technology for diagnosis and staging of primary breast cancer, offering superior sensitivity and specificity versus DBT alone. Deployed in tandem with DBT – in particular for women with denser breast tissue – CESM improves diagnostic accuracy through the injection of an iodinated contrast agent into the bloodstream (using the same protocol as for contrast-enhanced CT). In this way, the contrast agent enters general circulation but only enhances the region of clinical interest (a suspect lesion identified in the original mammogram) for subsequent imaging.
“The underlying principle of CESM is energy subtraction,” explains Remy Klausz, principal engineer for breastcare engineering at GE Healthcare Imaging. In brief, the CESM system acquires images at two different X-ray energies, with algorithms generating a recombined image – essentially subtracting the low-energy image from the high-energy image – to cancel out the images of background structures from regular breast tissue, enhancing areas of contrast uptake. “In this way,” Klausz adds, “CESM can demonstrate suspicious lesions otherwise ignored while significantly reducing false-positives – and without the considerable time and expense associated with follow-on functional imaging with breast MRI.”
Prioritizing the QA
From a commercial perspective, CESM continues to gain traction in the medical imaging community – though it’s also evident that not all mammography specialists are convinced. Some clinicians are uncomfortable with the injection of contrast agents in a mammography setting, while others are sceptical of CESM’s consistency – i.e. whether the modality will reliably demonstrate the presence or absence of iodine in suspect breast tissue time after time. Both, of course, are equally important: a suspicious area seen in the regular mammogram is likely to be non-cancerous if there is no contrast uptake, while enhanced contrast reveals a possibly cancerous lesion.
To address this cultural resistance, GE Healthcare has teamed up with CIRS, a US manufacturer of tissue-equivalent phantoms and simulators for medical imaging, radiation therapy and procedural training, to develop a simple yet comprehensive phantom for routine quality assurance (QA) of CESM systems. “Our motivation is to encourage more clinical users to adopt CESM,” explains Klausz. “Working with CIRS, we’ve come up with a versatile and easy-to-use phantom that, in just a few minutes, will give the radiologist full confidence that their CESM equipment is operating to specification.”
The original concept for the CESM phantom came from Klausz and his colleagues at GE Healthcare (see “Anatomy of a phantom”, below). After drafting the detailed technical specifications and concept drawings, Klausz approached the CIRS engineering management team with the idea for a joint development project. “CIRS is a leading provider of breast-equivalent phantom materials,” he adds, “so I had full confidence in their team’s ability to deliver what I was looking for.”
It took around six months from those initial discussions to first prototype, with CIRS granted full commercial rights to the phantom under the terms of the collaboration agreement. “Making a phantom is not our core competency at GE Healthcare,” explains Klausz. “Our motivation here is to provide a QA enabling technology to support wider clinical acceptance of CESM.”
Clinical and R&D customers can now purchase the resulting Model 022 CESM phantom on a commercial basis from CIRS. What’s more, the Model 022 uses the same frame geometry as the vendor’s DBT phantom, also some common parts, to further streamline QA protocols across DBT and CESM modalities.
Klausz concludes: “My recommendation would be to deploy the Model 022 phantom ahead of every CESM session. It’s just a simple visual check – no measurement is needed – to confirm that everything is working as expected from a machine point of view.”
Anatomy of a phantom
The Model 022 CESM phantom represents an average human breast in size and shape, demonstrating the presence or absence of iodine in tissues via different iodine concentrations and non-iodinated breast-tissue substitutes (see figure 1). The phantom consists of four slabs:
- A 10 mm thick target slab is made up of breast-equivalent materials representative of a 50:50 ratio of gland and adipose tissue. The slab contains two sets of four plugs, each set comprising plugs with clinically relevant iodine concentrations of 0.25, 0.5, 1.0 and 2.0 mg/cm2 A fifth plug, made of 100% glandular tissue-equivalent material, is positioned in the centre of each plug group to mimic a glandular lesion.
- The background slab, 25 mm thick, is made of two halves (100% adipose-equivalent material and 100% glandular-equivalent material) to test iodine separation from the background over a wide range of densities.
- The top and bottom slabs are 10 mm thick, made from 100% adipose-equivalent material, and have rounded edges to mimic the realistic shape of a compressed breast.
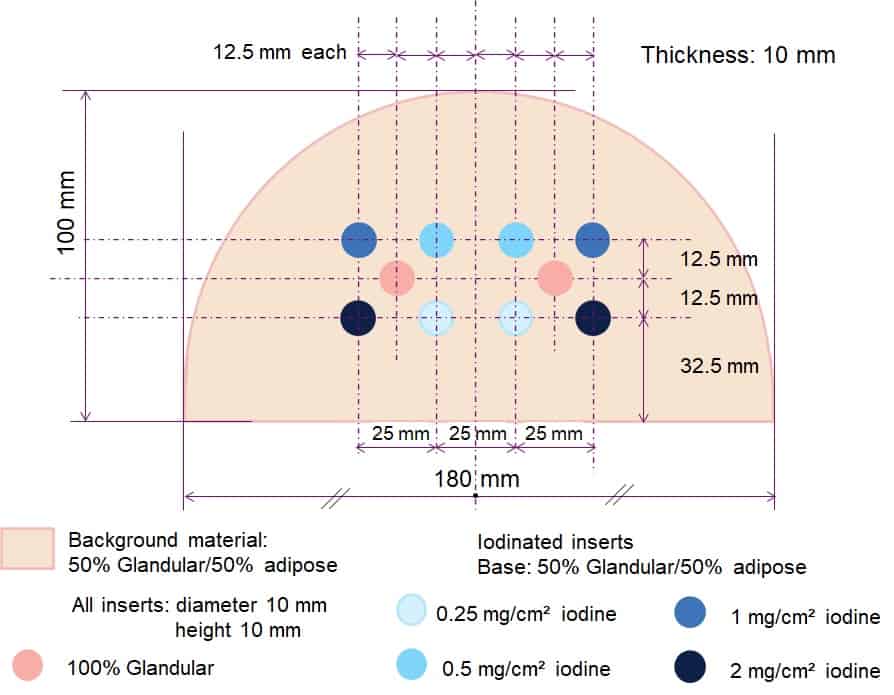
Fig 1: The layout of the Model 022 CESM phantom has been arranged to match the shape and mounting of the CIRS DBT QA phantom. A magnetic fixture allows for repeatable alignment and positioning. (Courtesy: GE Healthcare and CIRS)




