Popping open a bottle of champagne is one of life’s great delights, but how much do you really know about the science behind this greatest of wines? Gérard Liger-Belair reveals his six favourite champagne secrets

As the festive season approaches, many of us will look forward to popping open a bottle of champagne, whether it’s to celebrate Christmas with family and friends or to welcome in the new year. Next time you treat yourself to a bottle, your pleasure will surely be enhanced by a little scientific understanding of these fizzy, fine white wines. Champagne-making is an art form that has been refined over centuries, but thanks to advanced scientific instruments, we now know a lot about the subtle processes that give this legendary wine its sparkle to the eye, its tingle to the tongue.
As a physicist who studies champagne for a living, I can say that examining the bubbles in a glass of fizz is far from frivolous. Yes, the work has a pure, intrinsic interest, but it also has implications for other areas where bubbles play a role. In marine science, for example, we know that when an ocean wave breaks, it can trap air bubbles that burst when they reach the surface, ejecting aerosol droplets into the atmosphere – just like the bubbles in champagne.
So don’t feel guilty. Just sit back and enjoy my six scientific secrets of champagne – they’re bound to be a talking point when you next crack open a bottle. Oh, and before you ask where we get our champagne from, our university has its own small vineyard producing several hundred bottles per year. We also receive samples from various champagne houses that show an interest in our research, including Moët & Chandon, Veuve Clicquot and Pommery. My colleagues and I don’t drink the samples…if we did, we might never get any work done.
1. What’s that fog you see after popping open a champagne bottle?
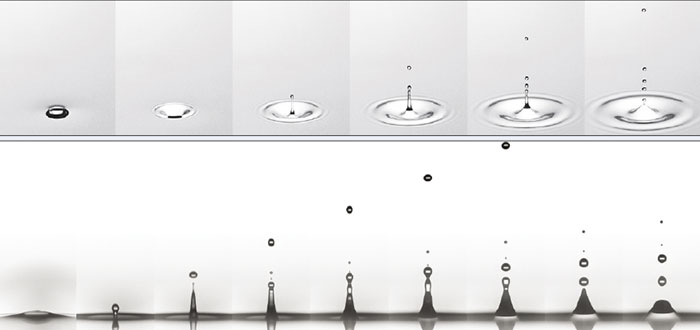
Before enjoying the pleasures of champagne, you obviously need to uncork the bottle so you can pour that lovely liquid out. Although it’s safer to do so by gingerly prising the cork out bit by bit, most of us will surely have popped open a bottle of champagne with a dramatic bang (see image series below). Look closely as the cork flies out of the bottleneck, however, and you’ll see a cloud of fog immediately forming. To understand how this beautiful effect is created, we need to remember that champagne is a mixture of water and ethanol, supersaturated with dissolved carbon dioxide (CO2).

The wines are created by first fermenting grapes harvested from the Champagne region of France in open vats. The CO2 is formed (along with further ethanol) during a second fermentation stage (the prise de mousse) that takes place after the wine has been transferred to hermetically sealed bottles. Champagne has special aromas too. These are volatile compounds that come from the grapes and the yeasts used for fermentation, as well as from the ageing process itself, in which those compounds slowly oxidize to create new molecules.
The amount of CO2 that gets dissolved in champagne is ruled by Henry’s law, which states that the equilibrium concentration of dissolved gas in a liquid is proportional to its “partial pressure” in the gas phase. (The partial pressure is the hypothetical pressure that one gas in a mixture would have if it alone occupied the volume of the mixture at the same temperature.) So when it comes to fizzy wine, Henry’s law means that getting lots of CO2 dissolved in the liquid requires a high pressure in the “headspace” between the wine and the cork. That’s why champagne bottles use tough glass and a tightly fitting cork.
But the amount of CO2 dissolved in champagne also depends on temperature, with the gas becoming much more soluble as the liquid cools. At 8–10 °C, which is the ideal serving temperature for champagne, there will usually be 11.5 g of dissolved CO2 per litre, while the pressure in the bottle is close to 5 bar. When you open a champagne bottle, the CO2 trapped in the headspace under the cork undergoes a huge drop in pressure from 5 bar down to an ambient pressure of 1 bar. Assuming the CO2 gas expands adiabatically (i.e. so fast that no heat exchange can occur), the gas cools by an incredible 80–85 °C, making water vapour and traces of ethanol vapour condense to create tiny droplets of fog (2013 J. Food Eng. 116 78).
Moreover, once the bottle has been uncorked, the thermodynamic equilibrium of CO2 is broken. The dissolved CO2 steadily escapes from the liquid because the partial pressure of CO2 in the gas phase has dropped to roughly 0.0004 bar, which is the partial pressure of gas-phase CO2 in the atmosphere. Thermodynamically speaking, all the dissolved CO2 must now escape from the champagne, though fortunately that doesn’t occur instantaneously. You’ll need several tens of hours for your champagne to go completely flat, but hopefully you wouldn’t be daft enough to let such a good wine stand around for so long.
2. What’s the best way to pour champagne?
So you’ve popped open your bottle of precious champagne. Now how are you going to pour the wine into a glass to preserve the precious fizz and get the bubbles to stay for as long as possible? My colleagues and I at the University of Reims Champagne-Ardenne investigated this question using two different serving methods. The first involved pouring champagne straight down the middle of a vertically oriented flute, while the other was to pour it down the side of a tilted flute.
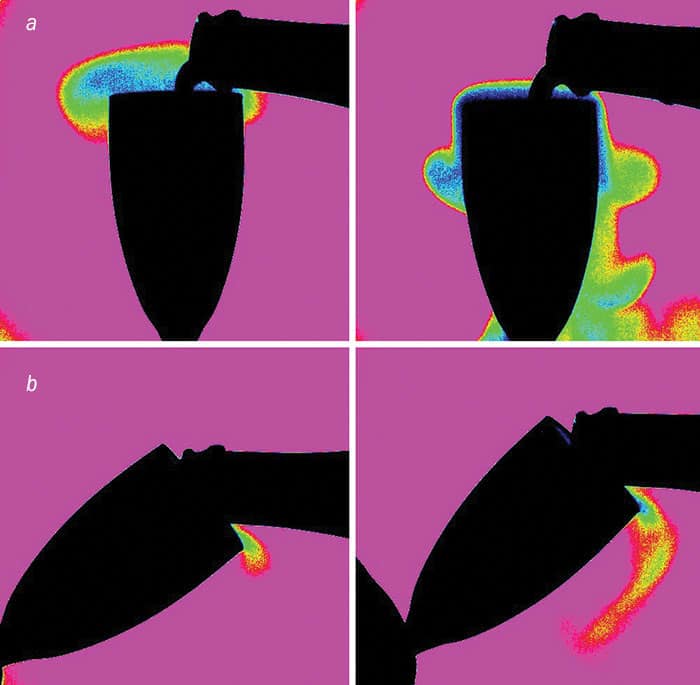
We found that tilting the glass, as you would when you pour beer, led to a much higher concentration of dissolved CO2 in the champagne than if the glass were kept upright. That’s because the beer-like way of serving champagne is much more gentle. It lets the champagne flow softly along the glass wall to progressively fill the flute. Pouring champagne straight down the middle of a vertically oriented glass, in contrast, creates turbulence and traps air bubbles in the liquid. Both effects force the dissolved CO2 to escape much more rapidly from the champagne.
These findings were corroborated using infrared imaging to visualize the clouds of gaseous CO2 that escape during the pouring process (figure 1). CO2 absorbs infrared light very strongly at wavelengths of about 4.2 μm, with the resulting images being brighter where there’s lots of the gas slopping about – down the sides of a vertical flute. My advice is to treat champagne a little more like beer – at least, when it comes to serving it. We also discovered that you lose less CO2 if the champagne is chilled because the wine gets more viscous as its temperature falls. But if the wine is warm, you get more turbulence and agitation in the champagne as it’s poured, forcing the dissolved CO2 to escape more rapidly from the champagne.
3. What’s better: flute or coupe?
For champagne lovers, there’s one question that has long been debated and mulled over in bars, restaurants and speciality wine magazines. What is the best type of glass to drink champagne from – a long, thin flute or a bowl-like coupe? With little or no analytical data having ever been brought to bear on this drinking-vessel dilemma, we decided to turn to science. One thing is clear: when you taste champagne from a glass, the gaseous CO2 and volatile aromatic compounds progressively invade the headspace above the vessel, slowly altering your overall perception of those smells.
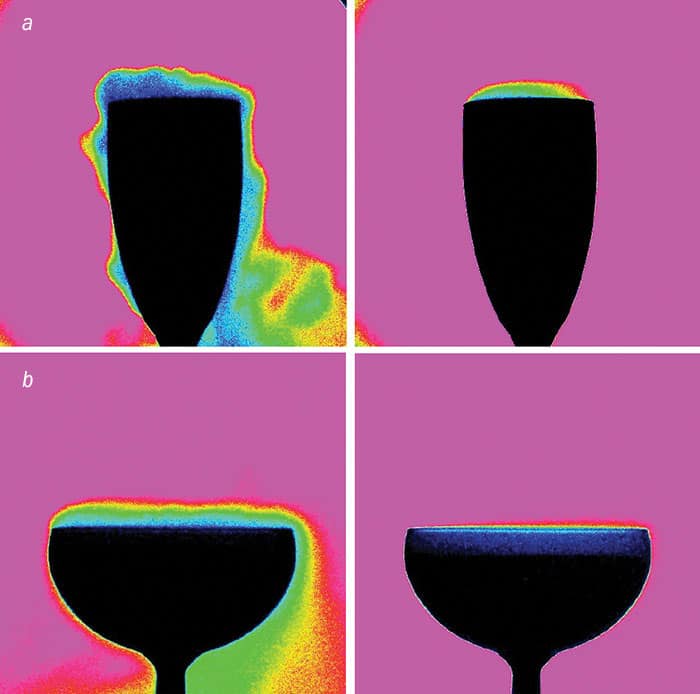
To find out how the geometry of the glass affects the drink’s smell, we measured the amount of gaseous CO2 and ethanol above the glass using gas chromatography. We found that when you pour champagne held at 20 °C into a flute, the headspace above the glass contains about 20% CO2 – roughly twice the figure above a coupe glass (2012 PLOS ONE 7 e30628). The tendency of flutes to hang on to concentrated quantities of CO2 was confirmed by infrared-imaging experiments. It appears that a narrow flute funnels the gaseous CO2 (and therefore the aromas) more effectively (figure 2a), whereas the broader coupe “dilutes” them (figure 2b).
Our findings chime with sensory analyses of champagne and other sparkling wines carried out by human tasters, who generally agree that the smell of champagne, and especially its first nose, is more irritating when champagne is served in a flute than in a coupe. Be careful not to sniff too deeply, as CO2 can burn or sting your nose if it’s too concentrated, especially if you’re drinking from a flute. In my opinion, a tulip-shaped wine glass (a bit shorter than a traditional flute and curved slightly inwards at the top) would be the best compromise between not having too much CO2 on the one hand yet having enough of an aroma on the other.
4. How many bubbles are there in a glass of champagne?
Knowing how many bubbles of CO2 are likely to nucleate in a glass of champagne is not just a question for sommeliers, wine journalists and experienced tasters. It’s also a fascinating question for any physicists wondering about the complex phenomena at play in a glass of fizzy wine. Thermodynamically speaking, a bubble has to overcome an energy barrier before it can form. In weakly supersaturated liquids, such as champagne or other carbonated drinks, bubbles don’t just pop into existence from nothing. To nucleate and grow freely, bubbles need pre-existing gas cavities above a critical radius of several tenths of a micron.

After using high-speed video cameras to examine wine glasses filled with champagne, we found that most bubble-nucleation sites are located on pre-existing gas cavities trapped within hollow, cylindrical cellulose fibres on the wall of the glass. Most of these fibres were either left behind by the towel we used to clean the glass or had been simply floating in the air before landing on the wall. Crucially, these fibres have an open tip that is several microns in size – exceeding the critical radius required for bubbles to grow.
To find out how many bubbles are likely to form in a glass, we developed mathematical models that combine the dynamics of bubble ascent with mass-transfer equations (2014 J. Phys. Chem. B 118 3156). As you might expect, the number of bubbles depends on both the wine and the glass, increasing with the temperature of the champagne and with the ambient pressure. The number of bubbles falls, however, with the height of the champagne, which means that if you like a lot of fizziness, don’t pour yourself too big a drink.
Roughly speaking, if you pour 100 ml of champagne straight down the middle of a vertically oriented flute, you’ll nucleate about one million bubbles – if you can resist drinking from your flute that is. Serving champagne by pouring it down the wall of a tilted flute, which keeps more CO2 dissolved, will yield tens of thousands more bubbles before the wine goes flat.
5. How do flow patterns in champagne affect its aroma?

After being created from tiny gas pockets trapped inside particles stuck on the glass wall, bubbles rise in a line towards the surface of the champagne. As the bubbles float up, they continue to get bigger by continuously absorbing molecules of CO2 dissolved in the liquid. These rising bubbles displace surrounding fluid, which in turn disturbs neighbouring fluid layers, dragging fluid particles in their wakes. As the champagne is constantly moving, with the surface changing all the time, volatile aromas can escape relatively easily, which is one of the joys of champagne. A flat, non-bubbly wine, in contrast, will be at rest in your glass. So unless you swirl it, the wine will quickly lose its smell.
Together with my colleagues Guillaume Polidori and Fabien Beaumont – both experts in fluid mechanics at Reims – I used laser tomography to reveal what the naked eye could not. Before pouring champagne into the glass, we first seeded the wine with tiny, approximately spherical “Rilsan” particles about 10–100 μm in diameter. Made from plastic, these particles have the same density as the fluid and so float in it, without rising or sinking under the effect of buoyancy. They reflect a lot of laser light, letting us visualize the flow patterns inside freshly poured glasses of champagne (figure 3).
In one test, we washed the glass using formic acid so that it was perfectly clean with no fibres remaining in it, which prevented any bubbles from nucleating. Free from effervescence, the champagne looked like a still wine with the Rilsan particles motionless. However, in a glass that has not been rinsed with acid, you do get effervescence, with a few nucleation sites giving rise to several “bubble trains” in the champagne. These trains make the champagne flow upward, creating vertically oriented streaks of light as the bubbles sweep the Rilsan particles along their path.

Flow patterns driven by ascending bubbles don’t just look pretty. They are a wonderful gift to the champagne taster, hugely increasing the diffusion of aromas above the champagne surface without having to lift a finger. In other words, there is absolutely no reason to swirl a glass of champagne or sparkling wine to enjoy the subtle mix of scents and flavours. The bubbles do the job for you.
6. Why do bursting bubbles make champagne have a better aroma?
The top of a flute filled with freshly poured champagne is a fantastic playground for exploring the physics behind collapsing bubbles. As an individual bubble reaches the surface of the liquid, it floats for a while like a mini iceberg, with only a tiny part of it emerging above the champagne surface. But when the emerged film of liquid disintegrates, a very complex hydrodynamic process ensues, causing the submerged part of the bubble to collapse. Together with Arnaud Antkowiak, Elisabeth Ghabache and Thomas Séon from Pierre and Marie Curie University in Paris, we studied this process using high-speed imaging combined with numerical modelling.
We found that when individual bubbles at the surface of champagne burst, they each produce a high-speed jet of liquid that quickly breaks up into several tiny champagne droplets (figure 4). In fact, the myriad of ascending bubbles collapse and spray a multitude of tiny droplets above the surface, creating wonderfully refreshing aerosols (figure 5). This characteristic champagne fizz releases far more flavours than you’d ever get from a flat wine.
Oceanographers have known for years that air bubbles trapped in sea water carry surface-active agents (or “surfactants”) that are released at the ocean surface when the bubbles burst, which led me and my team to wonder if champagne aerosols also have a high concentration of these molecules. Together with several friends and colleagues, led by Philippe Schmitt-Kopplin from the Department of Biogeochemistry and Analytics at Helmholtz Centre in Munich, we used ultrahigh-resolution mass spectrometry to analyse the chemical composition of champagne droplets (2009 Proc. Natl Acad. Sci. 106 16545).

We found that hundreds of different kinds of surfactant molecules get carried up and out of the liquid by ascending and bursting bubbles. They enter the champagne aerosols, which end up with a very different chemical fingerprint from the bulk champagne. Moreover, tens of these compounds, concentrated in the champagne aerosols, were identified as being the chemical precursors to aromas. By drawing a parallel between the fizz of the ocean and the fizz of champagne, our study revealed a relationship between bursting bubbles and the aromatic boost often attributed to champagne and sparkling wines. Our work supported the idea that rising and bursting bubbles act as a continuous “elevator” for aromas in each and every glass of champagne.
Champagne perfection
So there you have it. To enjoy champagne at its best, first chill the wine to the ideal serving temperature of 8–10 °C. Pour the champagne gently down the side of a tilted glass (not straight down the middle) so plenty of CO2 remains dissolved in the liquid. A tulip-shaped glass will give you the ideal balance between lots of aroma and not too much prickly CO2 getting up your nose. Don’t over-clean your glass or you won’t get enough of the bubble trains that help to release the champagne’s wonderful aromas by bringing lots of bubbles to the surface. And remember there’s no need to swirl champagne (as you would do with still wines) as those bubble trains will automatically disturb the liquid and help aromas escape through the surface towards your nose. Sniff deeply (but not too deeply) to enjoy the aromas, then let the lovely liquid into your mouth.
Season’s greetings!
- Enjoy the rest of the December 2015 issue of Physics World in our digital magazine or via the Physics World app for any iOS or Android smartphone or tablet. Membership of the Institute of Physics required