As global demands for carbon-neutral energy sources continue to rise, hydrogen gas (H2) produced by water splitting promises an ideal solution. Photocatalysts could absorb visible light, drive electron-hole movement and recombination, and subsequently produce hydrogen. Research has already optimized individual hydrogen reduction and water oxidation half reactions. However, combining both reactions in a single cell, a necessary step to make H2 gas production a feasible alternative energy resource, remains challenging. Researchers Christian Wolff et al., under the direction of Frank Wurthner and Jacek Stolarczyk, hope to address this challenge with their novel placement of two different co-catalysts on a cadmium sulphide (CdS) nanorod.
Room for two catalysts
Overlap of electrons and holes, proximity to their respective catalytic sites, and maintained separation are all critical factors within single-cell water splitting. It is also important to avoid close catalyst proximity, as this leads to premature quenching, preventing hydrogen gas production and ultimately lowering the overall unit efficiency.
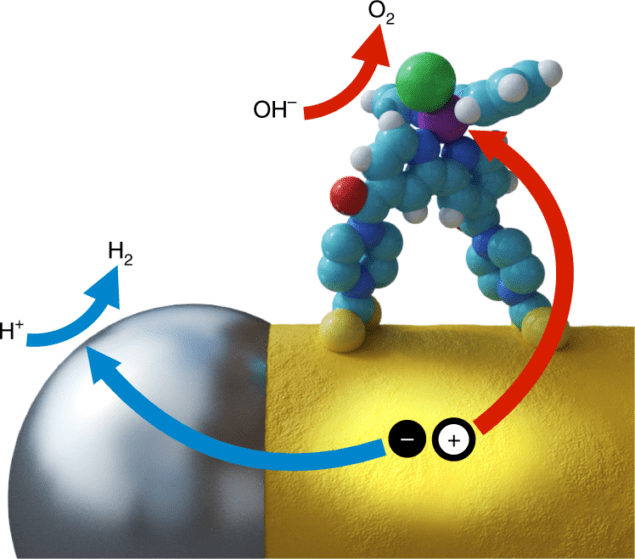
To overcome this difficulty, researchers at Wurzburg University and Munchen University placed separate reduction and oxidation catalysts at different locations on a semiconductor nanorod. Effective hydrogen-reducing platinum particles at the nanorod tips serve as “electron sinks” while the sides of the nanorods house novel molecular ruthenium oxidation catalysts. Wolff states, “The inherent anisotropy of the nanorod morphology is particularly well suited for such a process, providing two distinct, easily accessible and spatially separated types of site at the tips and on the side surface.”
Novel co-catalyst placement
In particular, intentional binding of each catalyst at the specific nanorod locations is critical. Wolff et al. used platinum thermal decomposition to place platinum at the ends of the CdS nanorods. Platinum is a well-known hydrogen reduction agent, as confirmed by photoluminescence analysis, which revealed, “fast electron transfer to the platinum, which strongly hinders radiative recombination.”
Water oxidation was more complicated. Specifically, the researchers utilized a ruthenium-based catalyst, which has previously demonstrated excellent hole movement. In water oxidation, though, these holes must still be separated from the electron-dense platinum coated tips. Wolff et al. accomplished this using functional dithiocarbamate groups on the ruthenium molecule. Via chemical ligation, the water oxidation catalysts anchored themselves on the surface of the nanorod. Photoluminescence then confirmed that the process deposited the catalyst in a monolayer.
Water splitting gas evolution characterization

CdS nanostructure excels for hydrogen generation
The doubly decorated nanorod demonstrated impressive hydrogen gas evolution. Samples displayed rates close to 16 μmol/hour and exceeded 20 μm/mol when in the presence of a sacrificial electron donor.
Oxygen gas evolution also fared well, although gaseous oxygen is inherently limited in water splitting. Deuterated labelling experiments confirmed that the oxygen evolution occurred from water splitting, meaning detrimental side reactions are likely not occurring. Additional gas evolution comparisons between unadorned and catalytically adorned nanorods demonstrate the usefulness of including both the platinum particles and ruthenium-based complex.
Full details are reported in Nature Energy.