“Sustainability” is the hottest topic in energy research today, but what does it actually mean? George Crabtree and John Sarrao describe what makes a technology sustainable, and outline the materials-science challenges standing between us and clean, long-lasting energy

The oil shock of the 1970s triggered worldwide awareness of oil dependency and launched a search for alternative sources of energy. But three decades on, these efforts have barely had an impact: oil still accounts for almost 40% of global energy use, and fossil fuels make up 85%. The US, for example, imported 20% of its oil in 1970; today the figure is 60%, and other countries import even larger fractions of the oil they consume. The problem of oil dependency is compounded by cost. Before the current recession, the price of oil peaked at $140 a barrel – five times its price in 2002 and 10 times its price in 1976 – rewriting the economics of transportation, food, manufacturing and trade that underlie the operation of society. In addition to dependency and cost, energy security is a pervasive threat. The concentration of oil production in a few regions of the world makes the supply of oil vulnerable to unpredictable events such as weather, terrorism, and geopolitical manoeuvring. Because oil provides so much of our energy, severe reductions in its flow would dramatically change the way we live.
The current outlook for energy adds a crucial new dimension that was not present in the first oil shock: carbon-dioxide emissions and climate change. The Intergovernmental Panel on Climate Change has documented global warming through rising sea levels, shrinking snow cover in the northern hemisphere and higher surface temperatures. These increases in temperature track similar increases in the concentration of carbon dioxide in the atmosphere – a remarkable correlation that extends over four ice ages covering the last 400,000 years. Carbon-dioxide levels are now 30% higher than they were before the Industrial Revolution, and they are rising at an accelerating pace, driven by the human combustion of fossil fuels. The potential implications for global warming and climate change are sobering. Left unchecked, climate change could produce dislocations in the agricultural, trade and demographic patterns that define global economic and social structures.
A particularly worrying feature of global warming is the timescale involved. It takes 400–1000 years for carbon dioxide in the atmosphere to equilibrate in the deep ocean. Hence, the carbon dioxide that we have already added – and continue to add – to the atmosphere will affect not only our grandchildren but also their grandchildren and many generations beyond. The long-term impact of global warming requires a sustained investment of intellectual resources to understand the dynamics of climate change, rather than the short-lived interest and spending surge that followed the oil shock of the 1970s.
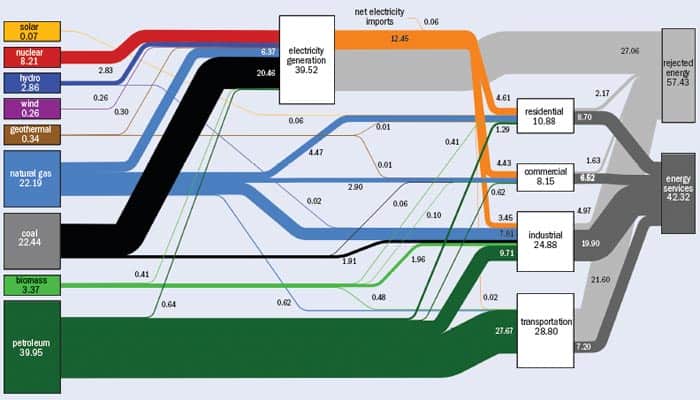
The dual challenges of energy and climate are captured in a single term: sustainability. Our current reliance on oil and other fossil fuels (see “Where the energy goes”) and our unfettered emission of carbon dioxide to the atmosphere are not sustainable activities. We are using oil and fossil fuels at far greater rates than nature creates them, and their cost and supply are fragile and volatile. Our carbon-dioxide emissions are growing by 22% per decade and they threaten to overwhelm the ability of the ocean–atmosphere system to absorb them and maintain a stable climate. These activities not only deplete the fossil resources required for our current energy system, but also undermine the environment and climate essential to our future prosperity.
The transition to sustainable energy technologies requires fundamental changes in the way we do business. Oil and emissions have become woven into the fabric of our economy and society, thanks to fossil fuels’ unprecedented success in delivering cheap energy when and where we need it. Changing these patterns will involve major disruptions of business as usual. Fun_damentally new ways of producing and using energy are needed, and they will require massive amounts of innovation in materials and chemical processes.
Defining sustainability
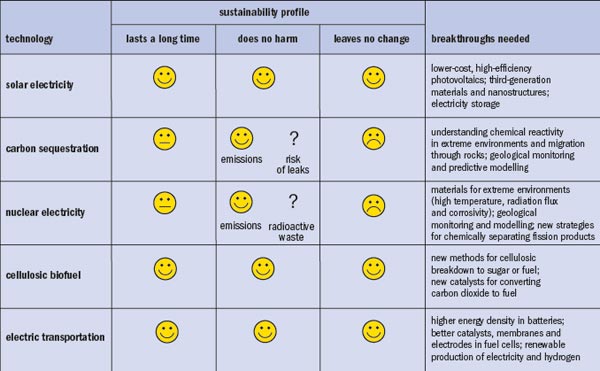
Although most people agree that more-sustainable energy technologies are desirable, they often find it harder to agree on exactly how sustainable these technologies need to be, and even precisely what is meant by sustainability. To clarify the debate, we suggest three criteria for sustainability, each of which captures a different feature of the problem. While we do not have the luxury of achieving full sustainability for all of our next-generation energy technologies, we can use these definitions to select our strategic sustainability targets and track our progress toward achieving them. As will become clear, the most sustainable energy tech_nol_ogies require the most challenging fundamental science breakthroughs (see “Making the grade”).
The first criterion for sustainability is “lasts a long time”. This quality has been a feature of many energy sources we have used historically, including wood in ancient times and oil throughout most of the 20th century. The definition of “long time” is, of course, relative: the world’s demand for energy long ago outpaced the ability of wood to supply it, and the production of oil is likely to peak sometime within the next few decades. Substantial reductions in the rate of oil consumption through higher-efficiency processes can significantly impact on how long non-renewable resources last. In applying the “long time” criterion, we need to distinguish between energy sources that are effectively limitless and those that are finite but, for the moment, adequate. The second criterion for sustainability is “does no harm”. Burning fossil fuels releases pollutants such as sulphur and mercury that endanger human health, as well as greenhouse gases like carbon dioxide that threaten climate stability. Some alternatives to fossil fuels have their own degrees of potential harm, including the underground migration and leakage of sequestered carbon dioxide and the hazards of storing spent nuclear fuel.
The third and most strict criterion for sustainability is “leaves no change”. When the material outputs of energy generation and use are recycled to replace the inputs, the chemical cycle is said to be closed and the chemical state of the world is unchanged. The process of converting renewable energy sources like sunlight and wind to carriers like hydrogen or electricity comes closest to fulfilling this restrictive definition. Fossil energy systems, in contrast, usually operate as once-through processes, irreversibly converting hydrocarbons to carbon dioxide and water. Some such systems could, however, be retrofitted to collect and recycle the combustion products to make new hydrocarbon fuel. If this process used the Sun as its energy source, fossil fuels, too, could meet this criterion.
Solar, wind and sequestration
Solar electricity comes close to satisfying all three criteria in the sustainability profile. In this electricity-generation method, a solar photon strikes a semiconductor photocell, excites an electron that travels through transmission lines to be used for, say, lighting, transportation or information processing, before returning to the photocell, where it replaces the hole left by the original electronic excitation. Once completed, the electron round trip does no harm and leaves no chemical change. However, although solar electricity may be fully sustainable in operation, it is not necessarily fully sustainable in the construction or disposal of its infrastructure – both steps require energy and emit carbon dioxide. These often-ignored full-life-cycle issues must be considered when evaluating the sustainability of energy technologies.
Despite the appeal of solar electricity, serious technical challenges block its widespread deployment. Before the reach of solar electricity can expand, its costs must fall below those of fossil electricity and must be low enough to attract the majority of future demand growth without artificial incentives. Achieving this will require breakthroughs in understanding and controlling the fundamental nano-scale phenomena of photo_excitation, charge separation and charge transport in, for example, high-efficiency multijunction solar cells and in low-cost organic and thin-film solar cells (see pp40–45, print edition only). An even greater and less-explored challenge is utility-scale electricity storage to bridge the day–night and cloudy–sunny cycles. Without the ability to store electricity, solar power can never be more than a supplement to fossil energy generation.
As a derivative of solar energy, wind electricity shares its sustainability profile, with the potential to satisfy all three criteria. The barriers to wind electricity are cost, utility-scale storage of electricity to bridge calm days and long-distance transmission capacity to deliver wind energy from its remote sources to urban population centres. The output of wind turbines is limited by the weight of the generator that can be supported on the tower. Superconducting generators can, however, lower the cost and land area required for wind electricity by a factor of two because they produce twice the output but are the same size and weight as conventional generators.
As for fossil-fuel electricity plants, they can be made more sustainable by capturing their carbon-dioxide emissions and sequestering the gas in underground geologic formations. Carbon sequestration prevents the carbon dioxide from entering the atmosphere – a positive step toward “doing no harm”. This positive step is balanced, however, by the challenges associated with injecting the carbon dioxide underground. We know little about how carbon dioxide reacts with the porous rocks in which it would reside, and less still about how far it might migrate during the thousands of years it must remain there. Carbon dioxide is supercritical under sequestration conditions, and the high temperature and pressure alter its reaction chemistry and enable it to diffuse quicker through porous rocks. These primary scientific challenges require a host of studies of surface-reaction chemistry to identify reaction pathways, intermediate species, chemical kinetics and diffusion phenomena under simulated sequestration conditions.
Beyond reaction chemistry, techniques for monitoring and modelling the migration of large quantities of supercritical carbon dioxide are also needed, so that we can anticipate where and how far it might travel over the thousands of years it must remain underground. The potential for contaminating an aquifer or finding an escape route to the atmosphere must be thoroughly understood for every sequestration site. Leakage is indeed one of the biggest challenges. A sequestration system with a leak rate of 1% per year exhausts all the carbon dioxide stored in its first year of operation in just a century – a blink of an eye on the timescale of ocean–atmosphere dynamics. During release, heavy carbon dioxide can displace lighter oxygen in low-lying areas, possibly leading to the suffocation of people and animals, as happened in a catastrophic incident at Lake Nyos, Cameroon, in 1986.
From a sustainability perspective, sequestration allows us to use the Earth’s coal resources (which will last longer than oil, though not as long as the Sun) with reduced harm to the atmosphere. Storing carbon dioxide underground, however, carries potential risks of contamination and leakage that are largely unexplored. Sequestration also leaves clear chemical changes as coal is removed from the Earth and carbon dioxide is injected.
The nuclear options
Like carbon sequestration, nuclear electricity keeps greenhouse gases out of the atmosphere, and thus represents a step towards sustainability. Next-generation reactors based on new materials could last longer than reactors designed in the 1960s – typically 80 years or more instead of 60 – and can turn 50% of the heat produced by fission into electricity, compared with 32% for existing reactors. Higher efficiency also allows the uranium supply to last longer; this, together with longer lifetimes, reduces the number of nuclear plants that will need to be built. These increases in efficiency can be achieved by operating reactors at higher temperatures (1000 K instead of the current 650 K) and at neutron fluxes an order of magnitude higher than the current values of 4 × 1013 n cm–2 s–1. However, at such high temperatures and fluxes, chemical corrosion is an additional serious challenge. Next-generation reactors will require a new generation of “extreme materials” that can not only survive, but also function under the triple extremes of high temperature, high neutron flux and aggressive chemical corrosion. Advanced ferritic steels hold promise in these environments; developing them by design rather than serendipitously will accelerate deployment significantly.
The scientific challenge for next-generation extreme materials – whatever their composition – is to understand their failure modes, and to prolong their useful lifetimes by interrupting or arresting these failures. Damage starts with atomic displacements that create interstitials and vacancies, which then migrate and aggregate to form clusters and ever-larger extended structures. Eventually, the damage reaches macroscopic dimensions, leading to degradation of performance and failure. This problem is massively multiscale, covering nine orders of magnitude in its spatial dimension, and neither experiment nor theory has yet captured this complexity in a single framework.
On the experimental side, in situ measurements of neutron irradiation with atomic or nano-scale resolution are needed to observe the initial damage processes, followed by coarser-grained experiments to capture migration, aggregation and ultimately macroscopic failure. The modelling challenge is equally dramatic: kinetic energy from an incident particle is transferred successively to electronic, atomic, vibrational and structural systems, requiring a diverse mix of theoretical formulations appropriate for different spatial scales.
To create new extreme materials that operate effectively in reactor environments we will have to go beyond observation and modelling to controlling the evolution of defect structures and interrupting their development before they can degrade performance. Introducing designer interfaces that collect and trap nano-scale defects before they cluster is one strategy for making materials defect-tolerant or self-healing. Treating defected regions with targeted photon or particle beams to anneal out the damage before it grows beyond a critical size is another. Although developed for nuclear applications, next-generation materials with these features should find wide application in other areas, including high-temperature turbines and coal-fired boilers, thus bringing overall energy efficiency closer to thermodynamic limits.
The sustainability profile of nuclear electricity shares many qualitative features with carbon sequestration. Like coal, terrestrial uranium resources will last a few hundred years, longer than oil but not as long as the Sun, and nuclear reactors emit no carbon dioxide into the atmosphere. The threat to the atmosphere is, however, replaced by a new potential danger: spent fuel that must be stored, perhaps underground, for hundreds or thousands of years to reduce its radiation level by natural decay. Developing fuels that fission a larger fraction of the available nuclei in a fuel rod – the current average is 4% – would mean that uranium supplies would last longer, and that the storage requirements for spent fuel would be less. Still, nuclear waste, like sequestered carbon dioxide, has the potential to leak, threatening water supplies and human health. New methods of treating, containing, monitoring and modelling nuclear waste are needed. Finally, like fossil-fuel electricity, nuclear electricity leaves substantial chemical change by removing uranium from the Earth and replacing it with radioactive waste.
Fusion electricity is more sustainable than fission because it replaces hazardous heavy-element fuel with benign, light and abundant hydrogen. The fusion product, helium, is likewise kind to the environment and climate. The fusion process, however, is even more extreme than fission, requiring neutron fluxes approximately 100 times greater than in fission reactors. Designing materials to withstand these exceptional irradiation conditions is a major scientific challenge.
Biofuels and electric cars
Replacing conventional oil with biofuels has the potential to achieve greater sustainability by recycling carbon dioxide and enabling fossil resources to last longer. However, it is now generally accepted that the energy balance and carbon footprint of corn ethanol and gasoline are only marginally different. In terms of lasting a long time and doing no harm, therefore, the two are approximately equally sustainable. Cellulosic ethanol made from the stalks and leaves of plants offers more hope. The scientific challenge for cellulosic ethanol is to discover or design a better chemical-conversion route from cellulose, nature’s evolution-hardened construction material, to fermentable sugar or liquid fuel. The known chemical and enzymatic routes are too expensive and inefficient to be competitive. Biofuels from algae offer an alternative route, since cultivating algae requires far less land than other biofuel crops, but a cost-competitive conversion route is not yet available and the science is still in its infancy.
Recycling carbon dioxide and water to produce fuel can also be done without biology, by using concentrated solar heat to drive high-temperature thermochemical reactions or electronic excitation from solar photons to drive photochemical reactions at room temperature. Both routes face materials challenges, and neither is scientifically ready to deploy. Thermochemical recycling requires advanced hybrid materials that can physically withstand and chemically promote the targeted reactions at high temperatures, and photochemical recycling requires new cost-effective catalysts that split carbon dioxide and water to drive the synthesis of hydrocarbon fuel. These challenges are firmly in the realm of discovery. Overcoming them will require use-inspired basic research (see “From observation to control”).
The sustainability profile of recycling carbon dioxide through biofuels (other than corn ethanol) and thermochemical or photochemical cycles is promising. These technologies can be fully or partially renewable and thus last a long time. They reduce harm to the environment by lowering greenhouse-gas emissions, and they have the potential to close the chemical cycle and leave no change.
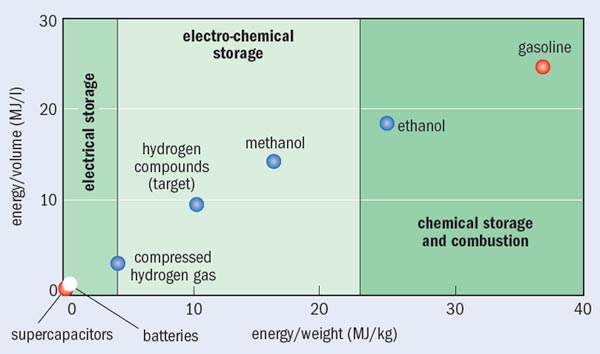
Electrifying transportation breaks the exclusive dependence of transportation on oil, thereby enabling flexibility in fuelling as more sustainable alternatives for electricity generation become available. Electric motors are also far more efficient (over 90%) and mechanically much simpler than gasoline engines, and so are able to transport people and goods at a much lower cost per mile. The challenge is onboard storage or generation of electricity to power the electric motor. For batteries, the energy density must be increased by a factor of two to five from current levels before the journey range of electric vehicles becomes competitive with multipurpose gasoline vehicles (see “How to store it”). The materials challenges are to develop electrodes for higher energy density and longer life-cycles; non-aqueous electrolytes for higher operating voltages; and entirely new electrochemistry approaches such as lithium–air electrodes or doubly ionized cations that can lower the battery’s charge-to-mass ratio.
Fuel cells offer an alternative to batteries by generating electricity onboard via hydrogen oxidation. Developing this alternative requires scientific breakthroughs in catalysis for the oxygen-reduction reaction at fuel-cell cathodes, high-density storage of hydrogen in lightweight solid compounds and the production of hydrogen from renewable resources. Substantial progress has been made in the last five years towards overcoming these barriers to using hydrogen as an energy carrier, as indicated by the rise in the number of researchers and published papers in the field. Such advances raise the probability that fuel cells will be a viable long-term option for transportation.
The sustainability profile of electric transportation is potentially high because electricity, once produced, is environmentally benign and leaves no chemical change. The primary sustainability issue is the large-scale production of electricity for battery-powered vehicles or of hydrogen for fuel-cell vehicles. Existing production routes use fossil fuels to generate electricity and the reformation of natural gas to produce hydrogen; both deplete finite natural resources and emit substantial amounts of carbon dioxide. In contrast, using renewable electricity produced by solar and wind to charge batteries or solar-powered water splitting to produce hydrogen has the potential to last a long time, do no harm and leave no change. Achieving these renewable production routes will require breakthroughs in discovery and use-inspired basic science.
Pick and mix
So which of these more sustainable energy alternatives should we develop? The expected doubling of global energy demand by 2050 is too daunting a challenge to be met by any single technology – we are likely to need many or even all of them. Some of the most sustainable options require significant scientific breakthroughs before they can be implemented. We can, however, follow a dual course of phasing in portions of sustainable technologies as breakthroughs make them cost-competitive, while aggressively pursuing the research needed to meet the remaining challenges and achieve even greater sustainability. In this framework, carbon sequestration, high-efficiency nuclear electricity and plug-in hybrid vehicles are near-term solutions that will precede the deployment of large-scale solar and wind power generation, utility-scale electricity storage and all-electric vehicles.
The scientific advances needed for these more sustainable energy technologies reflect a fundamental sea change in the role of materials in energy technologies. With traditional fossil energy, the important materials are the fuels, which are valued for their high energy content and low cost. Burning fuels to produce heat is the first step in the traditional energy-use chain. This heat is then converted by an internal combustion engine to mechanical motion for transportation or by a turbine and generator to electricity for a diversity of energy services.
Sustainable energy technologies, in contrast, tap into underused energy flows like sunlight or wind and use these to produce electricity or fuel directly, without a combustion step. The important components are the hi-tech materials and chemical processes that initiate and control the conversion of energy between photons, electrons and chemical bonds via nano-scale phenomena. Unlike the fuels of fossil-energy technology, which are valued as a raw commodity, the hi-tech materials of sustainable energy are valued for their ability to coordinate sophisticated nano-scale energy-conversion processes.
Many decades of advances in observing and modelling phenomena at ever smaller length scales and shorter timescales mean that science is poised to enter a new era where researchers will not only observe, but also control these nano-scale conversion processes. This new capability promises powerful breakthroughs in raising the efficiency and lowering the cost of sustainable energy technologies.
Breaking the scientific bottleneck
Despite 30 years of research and development, the deployment of sustainable energy technologies has hardly affected the global energy mix. The world is still over 80% dependent on fossil fuels, much as it was in 1970. The reason is remarkably simple: the cost of alternative energies is significantly higher than fossil fuels, and the energy enterprise, driven by economics, will always choose the lowest available cost. This simple fact clearly identifies the basic deployment challenge for sustainable energy: we must make fundamental scientific breakthroughs in materials and chemical processes, and exploit them to make sustainable energies cheaper than fossil fuels.
That we have not succeeded in doing so in three decades indicates the magnitude of the challenge. Existing sustainable technologies do not control materials and chemistry at the sophisticated level needed to cost-effectively convert sustainable sources into useful energy. But unlike fossil energy, which after a century of development operates near its theoretical maximum efficiency, sustainable energy technologies are still in their infancy. There is generous room for improvement in raising efficiency and lowering cost before intrinsic limits are reached; we are, in effect, where we were with the steam engine in James Watt’s day. Multijunction solar cells, for example, where two or more semiconductors tuned to different band-gap energies are connected in series, have a theoretical efficiency of over 50%, well above the 22% of the best single-junction silicon solar cells now in commercial production.
Indeed, silicon solar cells themselves symbolize the im_pact of materials breakthroughs on sustainable energy technologies. Their efficiency has risen from 6% in the 1950s to 22% today because of dramatic improvements in control of the purity, perfection and precision doping of silicon. Similar big improvements in efficiency and cost await other sustainable-energy technologies – provided we aggressively pursue scientific research to control the materials and chemical processes that govern nano-scale energy conversion. Discovering and designing these hi-tech materials and processes is the grand challenge of sustainable energy.
From observation to control
A remarkable string of advances over the last half-century has allowed us to probe and understand energy-conversion phenomena at ever smaller lengths and shorter timescales. Aberration-corrected transmission electron microscopy, a wealth of scanning probe microscopies, laser- and accelerator-based ultrafast photon pulses, intense neutron beams and massively parallel teraflop computing are all helping us to unravel the structures and dynamics of the macromolecules, proteins and complex materials architectures that carry out energy conversion on the nano-scale.
The next step is to not only observe, but also control these fundamental energy-conversion phenomena. To do this, researchers need to exploit the remarkable progress that has been made in nanoscience, numerical modelling and complex materials. Nanoscience has given us techniques for nearly atom-by-atom construction of complex atomic and molecular architectures, including “top-down” techniques such as optical and electron beam lithography and “bottom-up” approaches like molecular beam epitaxy, ink-jet printing and the rich potential of directed self-assembly – as exemplified by DNA-driven biological construction. These fabrication tools give us the means to make nano-scale structures with the complexity, precision and functionality of computer chips, which we now routinely manufacture on the micro-scale.
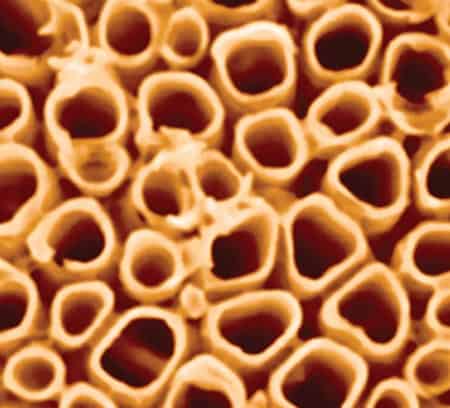
Nanotubes offer versatile and promising opportunities for controlling energy conversion at the nano-scale. TiO2 nanotubes like those pictured above are inexpensive, chemically inert, photostable, provide high surface-to-volume ratio and have band gaps that support sustainable energy technologies like solar water splitting, dye-sensitized solar cells and transparent conducting electrodes. They can be prepared by a variety of electrochemical processes, doped to tune their band gaps and decorated to promote surface catalytic activity.
On the modelling side, the past decade has seen computational speeds increase from teraflops (1012 operations per second) to petaflops, with exaflops on the horizon. These remarkable hardware advances allow the simulation of million-atom assemblies and the detailed nano-scale energy conversions they perform. The potential of this large-scale atomistic simulation is not just incrementally better, but game-changing.
With these tools in hand, our challenge is to design materials and molecular assemblies that can convert energy among photons, electrons and chemical bonds with minimum losses. One guiding example is nature. Green plants manufacture their internal nano-scale architectures from abundant, environmentally friendly elements and recycle them harmlessly to the environment at the end of their useful life. They split water and carbon dioxide at room temperature using sunlight and use the liberated carbon and hydrogen to synthesize sugar to fuel their growth and reproduction. Plants emit unwanted oxygen to the atmosphere, where it is recycled to carbon dioxide by respiration in animals and other living things, closing the chemical cycle and leaving no change. Cellulose in the leaves and stalks of plants is rich in sugars that can be converted to ethanol and other biofuels, provided we can break down the protective coating of lignin that shields cellulose from physical and chemical attack during its life. We can learn from biology by observing how lignin breaks down on the forest floor and how sunlight splits water and carbon dioxide in the growing plant, and adapt these processes to create and control our own sustainable hydrocarbon fuel cycle.
Another emerging guide is numerical materials simulation, which allows us to imagine complex nano-scale structures and then simulate their behaviour to see if they function as we intend. Such simulations dramatically shorten nature’s design process, which proceeds by incremental random mutations of existing structures. Once filtered and refined by computer simulation, the most promising nano-scale energy-processing structures can then provide inspiration for fabrication.
Complexity is an essential ingredient in any functional design. Like information processing, energy processing requires many sequential steps, including manipulating quanta of energy in photonic, electronic and molecular excitations, as well as chemical transformation. Not only must each step in the sequence be understood and controlled, but also the individual steps must be integrated into a seamlessly functioning assembly that efficiently links the functions of its constituent parts. Information technology and biology provide two shining examples of the value and impact of complexity on functionality. We have mastered the micro-scale complexity of information technology; the next frontier is the nano-scale complexity of biological function and sustainable energy technology.
At a Glance: Sustainability
- In order to be considered sustainable, an energy technology must last a long time, do no harm and leave the environment unchanged
- The technologies that come closest to meeting these criteria are also those that will require significant breakthroughs in materials science and processes to become cost-effective or viable on a large scale
- The key to achieving such breakthroughs lies in making the transition from observing materials and processes on the nano-scale to controlling these phenomena
- Biology is one source of inspiration for developing new technologies that are more sustainable and that will help solve the energy-climate problem
More about: Sustainability
V S Arunachalam et al. 2008 Harnessing materials for energy (special issue) MRS Bulletin 33 261–477
Basic Energy Sciences Advisory Committee 2007 Directing Matter and Energy: Five Challenges for Science and the Imagination; 2008 New Science for a Secure and Sustainable Energy Future www.sc.doe.gov/bes/reports/list.html
J Baxter et al. 2009 Nanoscale design to enable the revolution in renewable energy Energy and Environmental Sci. 2 559
M Eikerling et al. 2007 Driving the hydrogen economy Physics World July pp32–36
D Hafemeister et al. (ed) 2008 Physics of Sustainable Energy (Berkeley, 2008) (AIP Conf. Proc. 1044) (Melville, AIP)
Science 2007 Energy and sustainability (special issue) Science 315 737–815