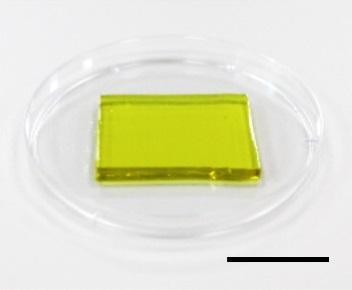
A new thermogalvanic hydrogel can simultaneously cool down electronic devices and convert the waste heat that they produce into electricity. The material, developed by a team of researchers at Wuhan University in China and the University of California Los Angeles (UCLA) in the US, decreases the temperature of a mobile phone battery by 20 °C and retrieves 5 μW of electricity at fast discharging rates. This reduced working temperature ensures that the battery operates safely, while the amount of electricity harvested is enough to power the hydrogel’s cooling system.
Many electronic devices – including solar cells and light-emitting diodes, as well as phone batteries – generate significant amounts of heat during normal operation. Not only is most of this heat wasted, it can also lead to localized overheating, which decreases the devices’ efficiency and lifespan. In some cases, the excess heat can even cause devices to explode or catch fire.
Traditional ways of recovering waste heat, such as thermoelectric modules, involve adding extra thermal resistance. Unfortunately, this additional resistance prevents heat from dissipating, and thus increases the temperature of the electronic device’s core components. Removing heat tends to consume energy, especially if additional equipment like fans or pumps are required. This apparent conflict means that while researchers have previously succeeded in recovering waste heat from electronic devices, and in efficiently removing it, they have never accomplished both at the same time.
Separate thermodynamic cycles
Thermogalvanic cells, which consist of an electrolyte solution surrounded by two inert electrodes, show promise in reconciling the competing tasks of removing heat and converting it to electricity. In such a cell, electron-transferring (redox) reactions convert heat energy into electricity. Since the solvent in the cell’s electrolyte solution is only present to support ion transport and electron transfer, it can undergo a separate thermodynamic cycle without affecting the heat-to-electricity conversion process. Hence, the water molecules in aqueous electrolytes can be allowed to evaporate and condense, completing a cycle of heat absorption and release that cools down the cell even as the thermal-electric conversion process continues.
A team led by Kang Liu of Wuhan University and Jun Chen of UCLA’s Department of Bioengineering has now developed a hydrogel film to accomplish this task. The hydrogel is based on a polyacrylamide framework infused with potassium, lithium and bromine ions, as well as the ferricyanide ions Fe(CN)63− and Fe(CN)64−.

Shear forces help make stretchable hydrogel
When heated, the ferricyanide ions transfer electrons between the cell’s electrodes, generating electricity. At the same time, confined water in the hydrogel is allowed to freely evaporate, which removes a large amount of heat without affecting the thermal-electric conversion process. The positive lithium and negative bromine ions serve to control the system’s moisture balance, facilitating water absorption from the surrounding air and thus “regenerating” the hydrogel.
Battery cooling
To show that their new hydrogel film could cool a real-world device, the researchers attached it to a mobile phone battery during fast discharging of 2.2 C (where C is a measure of the rate at which a battery is discharged relative to its maximum capacity). They found that some of the waste heat was converted into 5 μW of electricity and that the temperature of the battery deceased by 20 °C.
The researchers found that their film, which measured around 12 x 30 x 3.6 mm, is robust, with a mechanical strength of 0.24 MPa. They also showed that it can be stretched up to 2–3 times its original length without suffering any damage. Full details of the new thermogalvanic hydrogel are reported in Nano Letters.