
Molecules known as traction-force activated payloads (TrAPs) made from strands of DNA containing different chemical groups might be used to help heal wounds, according to new experiments by researchers at Imperial College London. The new technology may lead to the development of a new generation of materials that interact with damaged tissues to constructively promote the process of repair.
There are many examples of materials that are routinely employed to help heal wounds. These include collagen sponges that treat burns and scaffold-like implants that repair bones. “These materials act as passive bystanders, however, during tissue repair whereas wound healing is a highly dynamic, highly coordinated process involving many different cells over a period of time,” explains Ben Almquist, who led this research study. “TrAPs may provide the opportunity to design materials that ‘talk’ with these different cells in different ways and at different times to stimulate tissue repair processes.”
The damage caused by an injury triggers a series of natural defence and repair mechanisms that make cells migrate through the collagen networks present in a wound. As the cells move, they pull and exert traction forces on the collagen structure and this movement activates healing proteins that then begin their job of repairing tissue.
Recreating this natural process
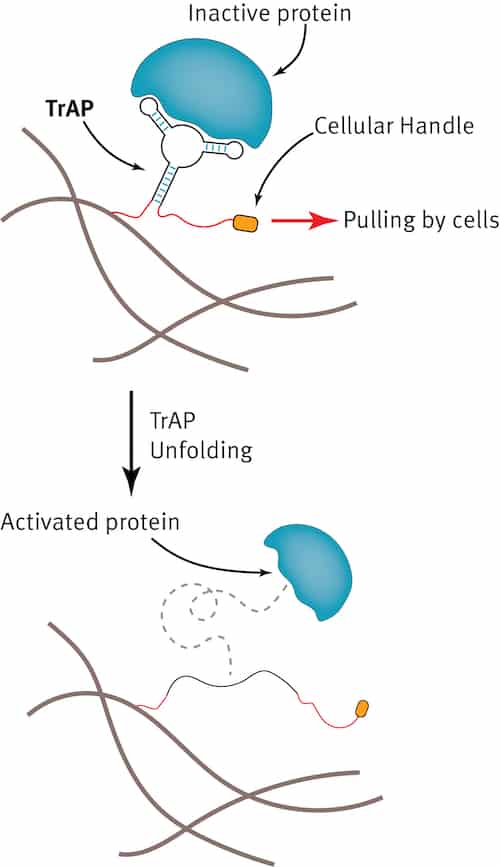
Almquist and colleagues designed their TrAPs as a way of recreating this natural process. The main component of the TrAPs is a short, single strand of DNA about 30-40 nucleotides long. Unlike in a double helix of DNA, in which two strands of DNA interact with each other in a specific way, the single strand can fold into a 3D shape by interacting with itself.
“When the DNA folds into this 3D structure, it can bind to proteins and inhibit them by fitting into small grooves or pockets on the surface of the protein,” says Almquist. “This is important since it is the 3D folded structure that can bind to proteins. These DNA molecules belong to a class of molecules called aptamers.”
To make their TrAPs, the researchers added a chemical group to one end of the single strand of DNA that they then used to attach the DNA to a material of interest, such as a collagen sponge. Next, they added a short peptide (a small fragment of a protein) to the other end of the strand. “This peptide is a like a ‘handle’ that cells can grab hold of,” explains Almquist.
Cells pull on the TrAPs
When placed in a cell culture, the researchers observed that the cells pull on the TrAPs as they travel through the collagen sponge. The traction forces produced unravel the DNA like a bow that unties to reveal and activate the bound proteins. These proteins then instruct the cells to grow and multiply – just like what happens in the natural biological process of wound healing.
And that is not all: by changing the cellular “handle”, the researchers show that they can change which type of cell grabs hold and pulls. This means that the TrAPs can be tailored to release specific healing proteins depending on which cells are present.
“We are currently exploiting the ability of these TrAPs to heal critical-size bone defects, for example,” says Almquist. “These are defects in bones that are too large to heal on their own.”
Reducing undesirable side effects
At the moment, therapeutic proteins are used to promote bone healing, but because of inefficient delivery, this technique requires many thousands of times more protein than the body naturally uses. This can lead to unintended consequences like bone growing in soft tissue where it shouldn’t. “We expect that our TrAPs will dramatically reduce the amount of protein needed to be effective, reducing these undesirable side effects, while also decreasing overall treatment costs since far less protein is needed.”
Other potential therapy areas include reducing scar tissue after heart attacks and repairing damaged nerves, as well as developing treatments for wounds that won’t heal using conventional techniques. One example is diabetic foot ulcers, says Almquist, which are the leading cause of non-traumatic lower leg amputations.
TrAPs are fully synthetic and relatively simple to make, which means that they could be scaled up to industrial quantities, he adds. They are also cheaper to make than structures made by protein engineering techniques.

From photo, to 3D model, through to wound healing
Transfer to the clinic
“The fact that we use aptamers to make TrAPs is also a point in their favour,” he tells Physics World. “A handful of these molecules have already been clinically approved (or are undergoing clinical trials). This means that the technology we have developed will be easier to transfer to the clinic.”
The researchers, who report their work in Advanced Materials doi.org/10.1002/adma.201806380, received a “blue skies” research grant from The Engineering and Physical Sciences Research Council (EPSRC) last year to test how well their platform can heal large bone defects. “This is a key step in bringing the technology to the clinic, and it has the potential to revolutionize how we use therapeutic proteins for wound healing,” says Almquist.
“Since there has never been a method before that exploits cells pulling on materials to release therapeutics, there is much that we don’t know about how to optimally design these TrAPs for maximal impact and efficacy. We are thus now busy working on better understanding the mechanisms behind this effect.”



