A new metal-organic framework material based on aluminium can store large amounts of hydrogen and methane at relatively low pressures. The material might be used to carry clean energy in fuel cell-powered vehicles according to the researchers at Northwestern University in the US who developed it.
In 2017, transport vehicles (including cars, trucks, planes, trains and boats) overtook power plants as the largest source of greenhouse gas emissions in the US. The share of transport-based emissions increased still further in 2018, and the trend is expected to continue – making the search for transport-friendly alternative energy sources ever more important.
Methane and hydrogen are often touted as potential replacements for diesel and petrol fuels in vehicles. While methane is considered a “transition” fuel, since its combustion still emits carbon dioxide (albeit less than that of petrol), hydrogen has been hailed as “the fuel of the future” since burning it produces neither carbon dioxide nor particulate-based pollution.
The problem is that because both hydrogen and methane are gases at ambient temperatures, they need to be compressed and kept at high pressures (of 700 bar and 250 bar respectively) whenever they are transported and stored. Since such high pressures can create hazards for drivers and others involved in handling stored fuel, the maximum storage pressure limit for real-world vehicles has been set to 100 bar – significantly reducing the amount of gas that can be stored in a given space.
Storing increased amounts of gas without increasing the pressure
In recent years, researchers have investigated high-surface-area porous adsorbent materials as a means of increasing the amounts of gas that can be stored in a given volume without increasing the pressure. Metal-organic frameworks (MOFs), which have surface areas of 2000m2/g or more, are considered promising candidates. These highly crystalline materials are made up of organic molecules and metal ions or clusters that self-assemble into multidimensional structures, and are easy to design thanks to their tailorable pore chemistry and shape.
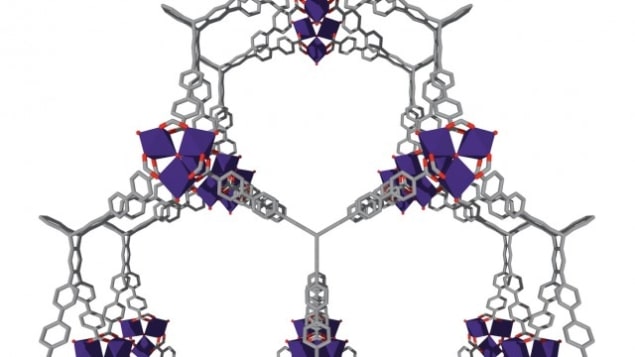
The Northwestern team, led by Omar Farha, used molecular simulations to inform the design of ultraporous MOFs based on trinuclear clusters, called NU-1501-M (where M is Al or Fe).
The researchers found that NU-1501-Al boasted high gravimetric (mass) and volumetric (size) storage performances for hydrogen and methane. Indeed, the material proved capable of storing 0.66 g of methane per gram of material at 100 bar and 270K – a value that exceeds the 0.5 g/g target set by the US Department of Energy (DOE) for developing the next generation of clean-energy automobiles. The material also has a high deliverable storage capacity of 14% by weight for hydrogen, which means it can store 14% of its own mass of hydrogen. While this figure seems low compared to its ability to store methane (66% by weight), it again surpasses the DOE target for 2020 of 4.5% by weight.
Storing gases with extreme efficiency in ‘crystalline sponges’
Nanosized pores mean a high surface area for gas adsorption
These high values are possible thanks to the material’s tiny pores, which measure less than 2.5nm across and thus offer a very high surface area for gas adsorption. As Farha notes, a one-gram sample of the material, with a volume equivalent to six M&M candies, has enough surface area to cover 1.3 American football fields.
This extensive surface area means that the team’s MOFs can store “tremendous” amounts of hydrogen and methane within their pores, Farha says, adding that the materials could deliver either gas to a car engine at lower pressures than are needed for current fuel-cell vehicles.
The new MOFs are detailed in Science.



