A new technique to synthesize radioactive upconverting nanocrystals that was previously used to extract uranium from ore could be used to make efficient radiotracers for biomedical imaging, say researchers at the University of Pennsylvania in the US. The nanophosphors, which emit beta-particles, could be ideal in theranostic applications and even targeted in vivo imaging when combined with CT scanning.
Upconverting materials emit light at a wavelength that is shorter than the wavelength of light they have been photoexcited with and are promising for applications in biomedical imaging. The so-called anti-Stokes shift in these materials limits the autofluorescence of nearby molecules within a sample. This significantly reduces background signals, allowing for better target detection.
Rare-earth compounds for upconversion
Although there are several materials and molecules capable of upconversion, rare-earth compounds are particularly good at converting near-infrared (NIR) light to visible light. These materials transfer the energy from the absorbed NIR photons in the form of excitons (excited electron-hole pairs) so that they are emitted with a higher energy (or shorter wavelength).
Another form of imaging commonly employed in nuclear medicine relies on unstable isotopes within radiolabelled molecules or nanoparticles (known as radiotracers) to produce a signal around the target. Radiotracers do not need to be externally excited, which allows the signals coming from them (beta-emission, for example) to penetrate deeper into tissue than optically-stimulated nanoparticles.
Beta-emission and upconversion in one system
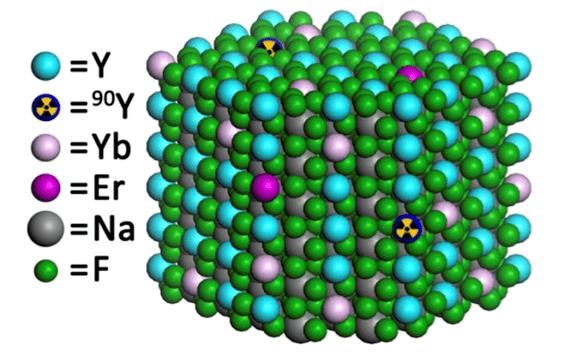
A team led by Christopher Murray made the nanophosphor sodium yttrium fluoride (NaFY) doped with the rare-earths erbium (Er) and ytterbium (Yb), and radiolabelled with 90-Y. This nanocrystal upconverts near-infrared light to the visible thanks to the rare-earth dopants and emits beta-particles thanks to the 90-Y.
The presence of both beta-emission and upconversion in one system is very rare in nature, explains team member and lead author this study Stan Najmr, and allows for deep tissue imaging because the two modalities are involved.
Hydroxide metathesis method
The researchers made their nanophosphors using a hydroxide metathesis method that was previously used to extract uranium from ore. “This method allows us to exchange one anion (for example, chloride) with another (trifluoroacetic acid), which is crucial for homogenously incorporating the radioactive element into the host matrix of the nanophosphor,” says Najmr.
The nanophosphors are produced through the rapid thermal decomposition of the rare-earth trifluoroacetates. “Once they have been synthesised, we silica coat them using the ‘Stober technique’. This allows us to disperse them in water, which is required for biomedical applications. Since these nanocrystals are fluorides, they are fairly inert to their environment.”
Extending the technique to other radioactive rare-earths
“This particle system is an excellent candidate for biomedical trials, especially in theranostics,” Najmr tells nanotechweb.org. “Combined with CT scanning and surface functionalisation, these nanocrystals could be used for targeted in vivo imaging.”
The researchers hope that their work will encourage other groups to consider this synthesis route for developing rare-earth nanocrystals and silica architectures. “For our part, we are eager to extend this technique to other radioactive rare-earths, such as lutetium (Lu) and samarium (Sm),” says Najmr. “These elements will provide new signals like gamma radiation that might lead to additional pathways for multimodal imaging.”
The research is detailed in in Nano Futures 2 025002.



