Type 1 diabetes results in the auto-immune destruction of insulin secreting β-cells in pancreatic islets. The disease is managed by insulin injections, but transplantation of pancreatic islets has been considered as a cure. However, re-vascularization of the transplanted islets is crucial for maintaining the function and viability of the β-cells, as it supports adequate nutrient and waste exchanges. Moreover, the interaction between the β-cells and the endothelial cells that line our vasculature is known to play an important role in supporting both cell types.
Recent research by Ibrahim Ozbolat and colleagues at Penn State University has highlighted how inclusion of endothelial cells into engineered pseudo-islets (termed EPIs) improves the viability and function of the EPIs, as well as promoting angiogenesis – the sprouting of new vasculature. The researchers utilized rat pancreatic beta cells (β-TC3) and rat heart microvessel endothelial cells (RHMVECs) to generate the EPIs (Biofabrication 10 035003).
To produce the pseudo-islets, the team generated a custom designed micro-mould to generate engineered 3D cell aggregates containing various ratios of β-cells to endothelial cells. These EPIs were then cultured for several days and characterized throughout to examine the effect of endothelial cells on β-cell function and viability.
The researchers found that EPIs with no endothelial cells added were fragile and had poor viability as the experiment progressed. However, EPIs formed from β-cells and RHMVECs in a 2:1 or 1:1 ratio showed increased proliferation, viability and insulin secretion.
Scanning electron microscopy allowed analysis of the surface morphology of the organoids. EPIs containing RHMVECs had a smoother surface topology and were more spheroidal, likely due to deposition of supportive extracellular matrix protein by the endothelial cells.
The team also used transmission electron microscopy to visualize insulin granules within the EPIs. Over the course of the experiment, these granules were seen to condense and form a well-defined membrane, evidence of the maturation of the cells.
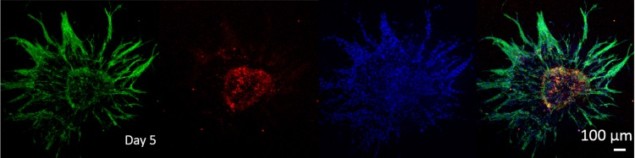
They then encapsulated the various forms of EPIs in a fibrin hydrogel, which was used to induce angiogenesis. Extensive angiogenic sprouting was seen in EPIs with endothelial cells, allowing fusion of EPIs in close proximity.
The researchers employed software tools to quantify the sprouting number and length for each EPI. Histology sectioning with haematoxylin and eosin staining showed the presence of hollow channels within the endothelial-loaded EPIs, while none were present in the β-cell only EPIs. The authors theorized that the channels formed by the introduced endothelial cells improved nutrient delivery, which helped improve viability in the EPIs.
The work from Ozbolat and his team has shown the benefits of 3D organoid culture of β-cells with endothelial cells. That angiogenesis was achieved throughout the pseudo-islets raises much hope for pancreatic tissue engineering. Indeed, appropriate vascularization is a large hurdle for bio-engineered tissue, as poor nutrient permeability and gas exchange can result in necrotic areas developing. This research is a step towards the ability to scale-up to larger tissue constructs, as the intrinsic vasculature may enhance diffusion throughout the structure, allowing generation of large, viable tissue structures. Scaled-up pseudo-islets like these could be used for drug screening and disease modelling of type 1 diabetes or, eventually, even clinical transplantation.



