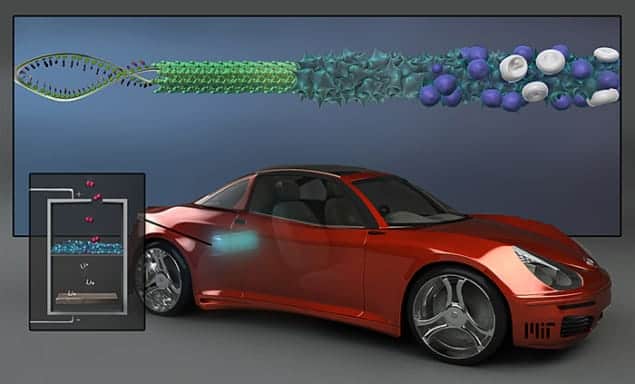
Research into lithium–air batteries that may in the future power electric cars and other electronic devices has just received a boost – from a virus. Scientists at the Massachusetts Institute of Technology (MIT) in the US have shown that using genetically modified viruses greatly increases the surface area of nanowires that work as electrodes in a battery’s cathode, thereby improving the battery’s charge-storage capacity.
A typical battery consists of a cathode, an anode (normally made of lithium), an ion conductor (or an “electrolyte”) through which charged ions flow easily, and a separator to keep the two ends apart. An electric current is produced as the positively charged lithium ions move from the anode to the cathode during cell discharge. When a battery is recharged, an external current makes the ions flow in the opposite direction, which results in the ions being stored at the anode.
Better batteries?
In recent years another type of battery, known as a lithium–air battery, has shown promise, and the development of these batteries could lead to electric cars being on the road for longer between charges. Such batteries oxidize lithium at the anode using a lightweight “air cathode” that replaces the much heavier metal-oxide cathodes used today. While the batteries hold much promise, at present traditional electrochemical capacitors perform much better – lithium–air batteries can only be charged and discharged a limited number of times before they start losing their charge-storing capacity. The batteries’ electrodes also need to be made from more durable and long-lasting materials.
Now, Dahyun Oh, a graduate student at MIT, and colleagues have found that increasing the surface area of the wire that acts as the electrode – thereby increasing the area where electrochemical activity takes place during the charging or discharging of the battery – could solve some of the above problems. The researchers also found that they could use a rod-shaped virus called M13 to grow the electrode materials – manganese-oxide wires that are a “favourite material” for the cathodes of lithium–air batteries. The virus is able to capture molecules of metals from water and bind them into various structural shapes. The scientists created an array of nanowires, each measuring about 80 nm across and “wrapped with very small amounts of nanoparticles of precious metal such as palladium or gold”, says Oh.
According to the researchers, their virus-crafted nanowires showed improved performance in lithium–air batteries because, unlike wires made using traditional chemical methods, the virus-built structures had a rough, spiky surface, which greatly increases their surface area. In addition, the viruses produced a 3D structure of cross-linked wires instead of isolated wires, thus giving the electrode greater stability. Angela Belcher, who is the lead researcher of the MIT team, likens the viruses’ growth to that of an abalone sea snail growing in its shell – it collects calcium from seawater and deposits it into a solid, linked structure.
Although the research is still in its early stages and such bio-batteries are not yet commercially available, the group does have a functional prototype that was tested through 50 cycles of charging and discharging. The scientists also used the biological approach to form functional materials “for solar cells, water splitting and cancer imaging”, according to Oh.
Environmentally friendly
Nanowire fabrication with viruses is simpler than most conventional methods – it can be carried out at room temperature and using a water-based process, instead of manipulating hazardous chemicals at high temperatures. And the process of building such bio-batteries uses no toxic or harmful materials, claim the scientists, making the batteries environmentally friendly. Traditional batteries contain heavy metals such as lead, mercury and cadmium. If they are not disposed of correctly, they end up in landfill and their toxic contents can enter the ecosystem.
Biology to the rescue
A number of the team members have been working on using viruses for bio-batteries for several years. In 2006, the researchers manipulated the genes inside a virus to form a nanowire anode a 10th the width of a human hair for a lithium-ion battery, followed by a study in 2009 where they also used viruses to build the cathode for the same lithium-ion battery.
But theirs is not the only research into bio-batteries. Electronics giant Sony has been looking into making such a battery, based on the proton–electron associated reaction and where glucose is used directly as electrodes, for the past decade. In addition, an Israeli start-up company called Store Dot claims to be “about five years away” from making the first commercially available bio-batteries. Its product is based on a new class of quantum dots – so-called nanodots of biological origin – that work as biological semiconductors and are able to store a charge, and can be used to make quick-charging, high-capacity batteries.
Using viruses for lithium–air and lithium-ion batteries as the medium for synthesis was “out-of-the-box thinking” says Jie Xiao at the Pacific Northwest National Laboratory, who was not involved in the study. She says that although it is still too early for the concept of lithium–air batteries to go to market, the research could “inspire other groups to work in this direction by adopting alternative approaches that may accelerate lithium–air-battery technology development”.
Take a look at the video below, courtesy of MIT, which illustrates how the wires for the batteries are grown.
The research is published in Nature Communications.