Silicon – the raw material of the microelectronics industry – can be developed into a biocompatible and biodegradable material that could lead to smaller, smarter and more-interactive implants in the human body.
“We can rebuild him. We have the technology. We have the capability to make the world’s first Bionic man.” So began each episode of 1970s TV show Six Million Dollar Man, as surgeons attempted to rebuild aeroplane crash victim Steve Austin by fitting him with bionic limbs and eagle-eye vision. As the popularity of the show proved, the concept of integrating man and machine has long fuelled our imagination. It has also been the subject of many other science-fiction movies and shows, including Bionic Woman, Robocop and Inspector Gadget.
Despite Hollywood’s somewhat over-the-top portrayals, our ability to develop miniature devices that can be implanted into the body has been quietly developing over the past 30 years. We can now produce precisely engineered and accurate electronic devices, such as pacemakers, that have extended the lives of thousands of patients. Much of the progress has been thanks to the enormous investment by the electronics industry in silicon technology, which has led to the evolution of an array of “intelligent” devices for human healthcare.
Cochlear implants, for example, were the first direct link between silicon chips and the human brain. When inductively coupled to an external microphone, the implant converts external sounds into electrical signals that are fed into an array of microelectrodes attached to nerves in the inner ear. The impulses are then passed by the auditory nerve to the brain, which interprets them as sounds. Cochlear implants are now so small that they have even been implanted in deaf toddlers, every one of whom has the chance to develop not only normal hearing but normal speech as well. Significant progress has also been made in developing silicon-based devices that can treat paralysis, blindness and neuro-degenerative disorders.
Healthy chips
However, the role of silicon in medicine has recently taken a new turn. Research by the authors during the past five years at the UK’s Defence Evaluation Research Agency (DERA) has identified forms of silicon that are not only valuable in the electronic sense as a semiconductor, but also in the medical sense as a “biomaterial”. In particular, we have found that so-called “porous” silicon – bulk silicon that has been deliberately riddled with nanometre-sized holes – can be biocompatible and biodegradable.
So rather than having to shield a silicon-based device from body tissues and the bloodstream, as has historically been the case, it is now theoretically possible to construct silicon-based devices that are genuinely “bioactive”. The surface of a chip could be designed so that it, say, interacts actively with living tissues in order to elicit some desirable physiological response. The silicon chip could, for example, stimulate bone-depositing cells in the body to cover the chip in both collagen and hydroxyapatite (the inorganic component of bone), thereby giving it a natural camouflage and enabling it to fuse with nearby bone. Other possibilities include tablets containing a cocktail of drugs hidden in tiny reservoirs that are released at different times (see below).
In an attempt to commercialize this research, we and our colleagues at DERA set up a company called pSiMedica in December last year with £1m of investment from the Australian company pSiVida and UK backers. Our aim is to build medical devices incorporating porous silicon and its variants. pSiMedica was, in fact, the first commercial DERA “joint venture” in the healthcare sector.
Nanostructuring silicon
So how can a lump of pure silicon be converted into biocompatible porous silicon? One popular technique is to etch the nanometre-sized pores into the surface of a silicon wafer using hydrofluoric-acid-based solutions (figure 1). Depending on the particular choice of wafer resistivity, electrolyte composition and applied current density, one can create “macropores” (with pore widths >50 nm), “mesopores” (pore width 2-50 nm) or even “micropores” (pore width <2 nm). The fraction of total volume that is void – the “porosity” – can be varied from about 1% to 95%.
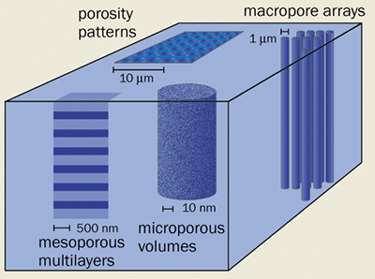
The highly porous silicon that is created in this manner is still pure silicon, but it behaves very differently to non-porous bulk silicon. Its “band gap” – the energy gap between the conduction and valence bands – can be more than twice that of bulk silicon, which increases its chemical reactivity and, remarkably, enables the silicon to emit visible light. Indeed, it is the light-emitting properties of porous silicon that have attracted the attention of most physicists to date, culminating in the recent reports of optical gain and stimulated emission (see further reading).
Unfortunately, the increased reactivity of porous nanostructured silicon is a problem in many optoelectronic applications. In particular, etching the silicon with acid produces unstable silicon-hydrogen bonds at the ends of the silicon skeleton. These bonds gradually oxidize in air, causing the silicon’s properties to change with time. For example, it becomes more electrically resistive.
Groups led by Mike Sailor at the University of California at San Diego, Jillian Buriak at Purdue University in Indiana and Jean-Noel Chazalviel at the Ecole Polytechnique in Palaiseau, France, have therefore been trying to modify the nanostructured surface of silicon. Their work has led to “derivitized” forms of porous silicon in which the silicon-hydrogen bonds are replaced by silicon-carbon bonds that do not oxidize. These processes could also enable a wide range of organic and biological molecules to be covalently bonded to the surface, which would give porous silicon a wide range of diverse properties. Linked antibodies, for example, could selectively bind targeted molecules (“antigens”) travelling through the bloodstream.
Bioactivity and biodegradability
The first tests to see how nanostructured silicon surfaces might behave in a biological environment were carried out in 1995. Our group at DERA showed that certain types of porous silicon – as well as “polycrystalline” silicon containing nanometre-sized grains – could stimulate and provide structural support for the growth of the inorganic component of bone (hydroxyapatite); the tests were carried out in a simulated biological “plasma” – the liquid part of body fluid (figure 2a). Originally developed by Tadashi Kokubo from Kyoto University in Japan, this simple test is now used by many biomaterial researchers to find out if ceramics can bond to bone in the body.

It was during such studies that the present authors made a striking observation. We noticed that thin layers of highly porous silicon could actually dissolve from the underlying non-porous wafer within a day or so. In other words, nanostructured silicon was shown to be biodegradable in vitro. This was a stunning result. It meant that the human body itself might be able to dissolve and excrete silicon. If so, silicon would then add its name to the roster of biodegradable materials. These are increasingly popular in medicine because they do not stay in the body forever and so reduce the risk of infection and of rejection by our immune system. In other words, biodegradable materials help the body to heal itself.
The behaviour of porous silicon in other simulated body environments – including the gastric juices of the stomach, intestinal fluid and the cerebrospinal fluid that bathes the brain and spinal cord – has now also been assessed in vitro (figures 2b – d). The results are promising. In almost all cases, underivitized porous silicon dissolves away relatively quickly, the one exception being gastric juice, which is very acidic and dramatically reduces the rate of biodegradation. This work could lead to “smart pills” that – once swallowed – deliver potent drugs to the colon.
Despite the thousands of in vivo tests that clinical scientists have carried out to develop and understand the biomaterials used by surgeons, there have been amazingly few published papers on bulk silicon and – until recently – none at all on the nanostructured forms of the material. The dearth of published information on the biocompatibility of silicon prompted us to investigate this aspect by conducting a major six-month study of both porous and non-porous silicon in guinea pigs. Performed according to ISO standards at DERA’s Biomedical Sciences Department, both forms of the semiconductor were found to have just as good tissue compatibility as titanium, a tried-and-tested biomaterial. While the bulk silicon and titanium hardly corroded at all over the six-month implantation period, the partially porosified silicon disks continuously decreased in weight, and became more and more corroded with time. This was the first demonstration that a semiconductor can be made simultaneously biocompatible and biodegradable.
So what are the possible uses for biodegradable silicon, bearing in mind the wide range of polymers, metals, ceramics and composites that are already available to the medical and pharmaceutical industries? The key features that distinguish silicon from other biomaterials are that it can be micromachined, it is a semiconductor, and it has an inert, crystalline form. Add to the menu the fact that porous silicon is biodegradable and compatible with human tissue and one has a highly versatile material from which implantable and intelligent devices can be built.
Its micromachinability enables precise yet complex shapes on the micron length scale to be mass produced. Its semiconducting properties, meanwhile, enable porous silicon to form part of a compact electrical microsystem with sensors, actuators and circuitry that can control biological activity, process incoming data and relay that information via biotelemetry to the outside world. Moreover, the level of porosity can be controlled, enabling porous silicon to be used in a wide variety of different clinical areas.
In diagnostics, for example, derivitized porous-silicon mirrors placed just under the skin could be used in minimally invasive optical monitoring of biochemical markers for cancer. And in biofiltration, porous-silicon boxes could protect insulin-secreting cells from the immune system of diabetic patients. Their pores would be made large enough to let nutrients in and insulin out, but small enough to stop the patient’s cells from attacking the boxed-in foreign cells. pSiMedica is, however, focusing its technology on two key areas: controlled drug delivery and orthopaedics/tissue engineering.
Intelligent and passive drug delivery
In its simplest form, a nanostructured form of porous silicon acts as a biodegradable “scaffold” that encapsulates a particular drug. The drug – in a suitable liquid form – could initially be drawn into the porous chip by capillary action. Once the chip has entered the body, the outer surface of the silicon scaffold will erode and the drug will gradually be released. The wonderful advantage of silicon is that it can be processed into all the forms that are currently used to administer drugs. It could be made into tablets for swallowing, “patches” that would deliver the drug through the skin, “microparticles” that would be injected, or “cylinders” that would release the drug slowly following injection into subcutaneous fat.
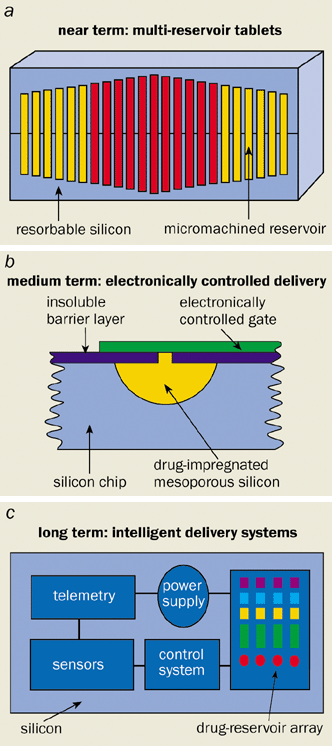
The growth in the number of ways of delivering drugs into the body stems from the increasing desire for drugs to be given at just the right dose and only to the appropriate part of the body. Chemotherapy drugs for cancer treatment, for example, only work without unacceptable side effects over a narrow range of concentration in the body. Jeff Coffer from Texas Christian University in the US has therefore been incorporating such drugs (cis-platin and carbo-platin) into the hydroxyapatite coating of porous-silicon chips, in an attempt to improve their delivery to bone tumours. The rate of release of the drug from the porous silicon can itself be controlled by varying the level of porosity in the silicon: highly porous silicon degrades faster than lightly porous silicon and therefore releases the drug faster. In other words, the rate of release of an active drug from a drug-impregnated porous-silicon chip can be adjusted by choosing the right microstructure. Another possibility is a multi-reservoir tablet that can release a cocktail of drugs at pre-determined times (figure 3a).
The rate of release can also be controlled by derivitizing the surface of the porous silicon – in other words, by replacing the hydrogen atoms at the ends of the silicon skeleton with other groups, such as amino acids or hydrophilic chains of carbon atoms. This is, perhaps, the first step towards the “smart” control of drug release. One could, for example, imagine drug molecules connected to a biodegradable silicon scaffold by bonds that are sensitive to a particular enzyme. The silicon would only release its drug when the enzyme meets that chip. The implant would then have a crude “measurement and response” function, triggering a cascade of biochemical events that maintains physiological requirements for the drug. Thomas Laurell and his group at Lund University in Sweden, who are taking advantage of the large internal surface area of porous silicon to make in vitro bioreactors, have already shown that enzymes can work well within porous silicon.
Of course, the above structures do not harness the full value and potential of biosilicon technology. With the continued miniaturization of electronic components – including power supplies, transducers and sensors – it is only a matter of time before more subtle regulation will be possible with this new biomaterial. Possibilities include “ticking tablets” that release their drug payload at a particular time (figure 3b). The holy grail is for drug release to be linked to in vivo diagnostic devices and computerized data collection (figure 3c). Doctors would then be able to fine-tune “disease management”, with substantial benefits for patients.
Orthopaedics and tissue engineering
The other key area of interest for pSiMedica is in orthopaedics and tissue engineering. Indeed, one of the first applications of artificial materials in the human body involved the internal reconstruction of damaged bone with metals. Materials that are favoured today include steel, titanium, polyethylene, ceramics, alloys of cobalt and chromium, as well as bioglasses and composites. There are, however, a number of fundamental limitations to the use of, say, titanium or steel implants. First, they often have to be removed after they have fulfilled their purpose. Second, bone tissue is actively growing and remoulding, which means that the metal does not always anchor itself properly to the bone. Another problem is that metal adheres poorly to bone in any case. Adhesive cements are therefore needed for knee and hip implants.

As a potential orthopaedic material, bone-bonding silicon has some clear attributes as well as drawbacks. It has a better tensile strength than steel, but – unlike metals – it is brittle and subject to impact damage. However, its Young’s modulus of elasticity can be tuned by varying the level of porosity to match that of either cortical (i.e. hard) or cancellous (i.e. spongy) bone. This is important to avoid the problem of “stress shielding” that often occurs when metallic prostheses are in direct contact with bone. (Essentially, bone is a tissue that thrives on stress, which means that any implants that take all the load will cause nearby healthy bone to die.)
But the biodegradability of porous silicon is perhaps the biggest advantage of this material. Renowned tissue engineers like Joseph Vacanti from Harvard Medical School in the US have already started using micromachined silicon moulds as “templates” to create precise three-dimensional topographies in biodegradable polymers. So why don’t we try making 3D structures from biodegradable silicon itself? A porous silicon structure could, for example, be deliberately sculpted to provide bone-building cells with a scaffold that they can penetrate and anchor to (figure 4). As the bone tissue deposits itself onto the scaffold, the porous silicon would slowly dissolve away – eventually leaving just new bone. The other advantage of porous silicon is that – unlike biodegradable ceramic or polymer scaffolds – it can conduct electricity. (Bone itself is piezoelectric, which has already led to electrical techniques for repairing broken bones that have failed to heal with other methods.)
Biocompatibility and manufacture
The fact that porous silicon corrodes away in the presence of biofluids is not quite enough for it to be labelled a true “biomaterial”. To earn that title, we have to be sure that it is non-toxic and biocompatible. We also have to know that the products it degrades into are safe and that they are properly excreted from the body. Fortunately, porous silicon degrades mainly into monomeric silicic acid (Si (OH)4), which just happens to be the most natural form of silicon in the environment. (Indeed, silicic acid accounts for 95% of the silicon that is cycled through rivers and oceans, and is present in many foods and drinks.) Furthermore, tests using radio-labelled silicic-acid drinks given to human volunteers resulted in the concentration of the acid in the bloodstream rising only very briefly above typical values of ~1 mg l-1. Urine excretion of silicic acid is also highly efficient and expels all the ingested silicon. In fact, the human body actually needs silicon in this form as an essential trace nutrient. Groups such as those led by Jonathan Powell of St Thomas’ Hospital in London are trying to find out why.
A wide range of in vitro tests are now also under way to reveal and quantify any potential sources of toxicity to different types of living cells grown directly on the material. We know, for example, that freshly etched layers of porous silicon can, as a result of hydrolysis, emit silane gas at concentrations of several parts per million and that the more reactive layers might supersaturate the nearby fluid with silicon. We have also found that bacteria, such as E-Coli, can readily colonize silicon chips, just as they are colonized by mammalian cells. We are therefore developing ways of sterilizing porous silicon so that its properties don’t change and that any risk of infecting the body is minimized.
What about the practicalities of manufacturing porous-silicon products? Thanks to its starring role in the microelectronics industry, we now know an enormous amount about how to process silicon. This practical know-how, which has been built up over the past 50 years, gives silicon a substantial advantage as a possible new healthcare material. After all, silicon is produced with a purity that would be the envy of most pharmaceutical companies. Perhaps such purity will not be required for many medical applications, but the fact that silicon is routinely handled in clean-room conditions and that many established processing techniques exist will undoubtedly spur on its contribution to medicine.
Indeed, at a purpose-built factory in Japan, Takao Yonehara of Canon Inc. directs the anodization of 10 000 wafers per month, as a way of supplying “silicon-on-insulator” (SOI) wafers, which are used for specialist chip applications. In this technique, layers of pure silicon are epitaxially deposited onto the porous upper layer of a silicon wafer. The wafer is then bonded to another wafer with an insulating silicon-oxide surface, so that the epitaxial silicon ends up sandwiched in the middle. The porous silicon is selectively dissolved away to leave the desired SOI product: epitaxial silicon on top of an oxide layer on top of the silicon wafer. The analogy is clear: Canon uses porous silicon as a sacrificial layer outside the body, while we at pSiMedica intend to use it within the body. The Canon enterprise has shown that nanostructuring silicon wafers in this manner can meet manufacturing requirements of scale-up, low cost, yield and clean-room compatibility.
A more bionic future?
We are but a few steps down the path to making machines man’s best friend. Implantable and interventional therapies are, of course, now commonplace. Market leaders such as Medtronic Inc. of the US have revenues of about $3bn and a broad portfolio of implantable products, including pacemakers, catheters, perfusion systems, drug-delivery devices, and systems for neurological and spinal benefit. Such systems are mainly based on electronic devices that are quite isolated from the biological systems into which they feed.
One particularly challenging – but potentially rewarding – research area that a number of groups are pursuing is the use of microelectronic implant technology to restore vision to the blind. Similarly, functional electrical stimulation to restore movement in paralysed patients is receiving much attention. Clearly, the application of electronic intelligence to many devices has been commercially fruitful and improved the quality of life for many patients.
So where do we go next? To satisfy commercial pressures, pSiMedica will begin by developing simple, relatively unintelligent drug-delivery devices. In the longer term, we want to develop implant systems that contain both technical and biological components, such as simple bio-interactive porous-silicon chips that can deliver drugs only when required by the body. The mechanics of such implant systems will, however, require many years of R&D before they reach the market.
As far as patient acceptability is concerned, we have already entered an era where people are generally comfortable with the idea of implants that can regulate key physiological activities, such as pacemakers to control irregular or slow heartbeats. Improved drug delivery using novel devices is also common, although most devices on the market neither boast nor require electronics at this stage. The biggest ethical challenge will perhaps come with the introduction of microelectronic monitoring devices that constantly tell doctors how a patient or their medication is performing. However, there have already been several successful products in this area, including an insertable “loop recorder” that was launched by Medtronic in 1998 to record a patient’s heart rate and rhythm non-stop for a year. It is likely to be a precursor to a range of minimally invasive devices for monitoring patient well-being. The early diagnosis of cancer recurrence would be one such area.
Nature does not create ultra-smooth planar surfaces like those of the polished wafers that populate silicon foundry lines. The living materials in our body rely on porosity to function properly, from the nanometre-wide pores of each cell membrane, to the architectures that govern interchange in tissues and organs. Is porous silicon an obvious “biomaterial bridge” between the basic requirement for a biocompatible and biodegradable material and the well established electronics arena? Time will no doubt tell – and will decide if chips will keep not only the grim reaper, but also the doctor away.