A photothermal, nanoparticle-based deep brain stimulation (DBS) system has successfully reversed the symptoms of Parkinson’s disease in laboratory mice. Under development by researchers in Beijing, China, the injectable, wireless DBS not only reversed neuron degeneration, but also boosted dopamine levels by clearing out the buildup of harmful fibrils around dopamine neurons. Following DBS treatment, diseased mice exhibited near comparable locomotive behaviour to that of healthy control mice.
Parkinson’s disease is a chronic brain disorder characterized by the degeneration of dopamine-producing neurons and the subsequent loss of dopamine in regions of the brain. Current DBS treatments focus on amplifying dopamine signalling and production, and may require permanent implantation of electrodes in the brain. Another approach under investigation is optogenetics, which involves gene modification. Both techniques increase dopamine levels and reduce Parkinsonian motor symptoms, but they do not restore degenerated neurons to stop disease progression.

The research team, at the National Center for Nanoscience and Technology of the Chinese Academy of Sciences, hypothesized that the heat-sensitive receptor TRPV1, which is highly expressed in dopamine neurons, could serve as a modulatory target to activate dopamine neurons in the substantia nigra of the midbrain. This region contains a large concentration of dopamine neurons and plays a crucial role in how the brain controls bodily movement.
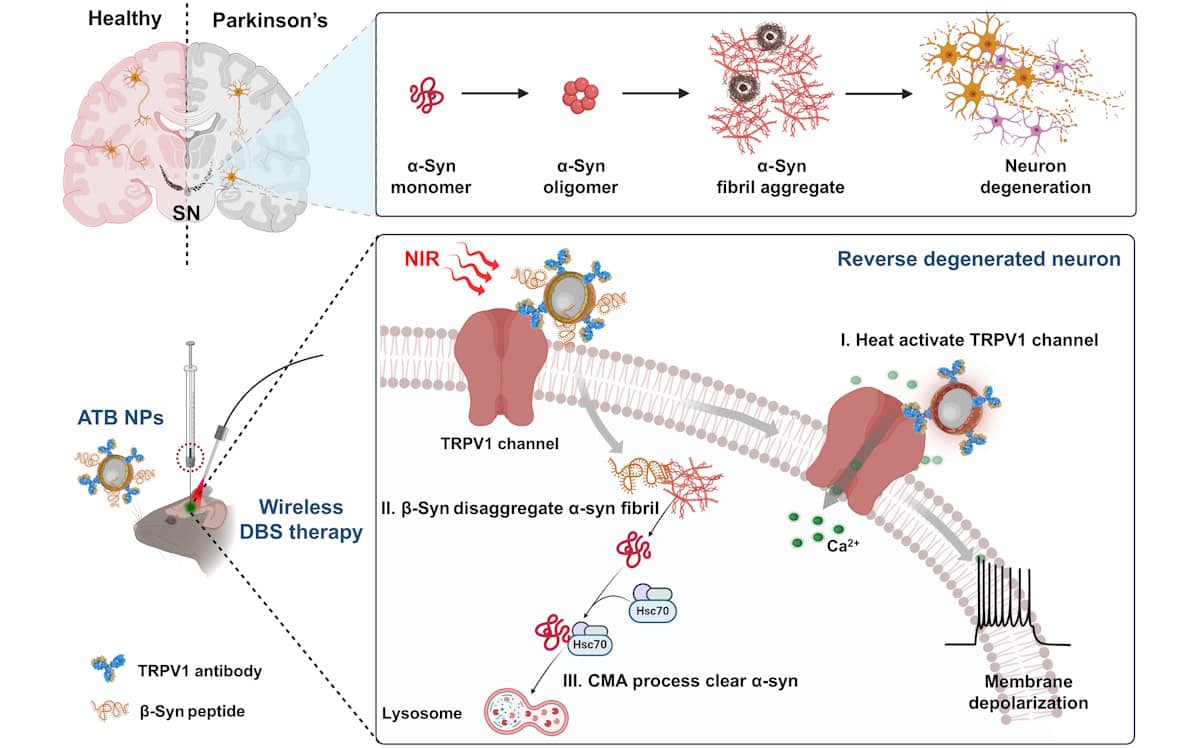
Previous studies have shown that neuron degeneration is mainly driven by α-synuclein (α-syn) fibrils aggregating in the substantia nigra. Successful treatment, therefore, relies on removing this build up, which requires restarting of the intracellular autophagic process (in which a cell breaks down and removes unnecessary or dysfunctional components).
As such, principal investigator Chunying Chen and colleagues aimed to develop a therapeutic system that could reduce α-syn accumulation by simultaneously disaggregating α-syn fibrils and initiating the autophagic process. Their three-component DBS nanosystem, named ATB (Au@TRPV1@β-syn), combines photothermal gold nanoparticles, dopamine neuron-activating TRPV1 antibodies, and β-synuclein (β-syn) peptides that break down α-syn fibrils.
The ATB nanoparticles anchor to dopamine neurons through the TRPV1 receptor then, acting as nanoantennae, convert pulsed near-infrared (NIR) irradiation into heat. This activates the heat-sensitive TRPV1 receptor and restores degenerated dopamine neurons. At the same time, the nanoparticles release β-syn peptides that clear out α-syn fibril buildup and stimulate intracellular autophagy.
The researchers first tested the system in vitro in cellular models of Parkinson’s disease. They verified that under NIR laser irradiation, ATB nanoparticles activate neurons through photothermal stimulation by acting on the TRPV1 receptor, and that the nanoparticles successfully counteracted the α-syn preformed fibril (PFF)-induced death of dopamine neurons. In cell viability assays, neuron death was reduced from 68% to zero following ATB nanoparticle treatment.
Next, Chen and colleagues investigated mice with PFF-induced Parkinson’s disease. The DBS treatment begins with stereotactic injection of the ATB nanoparticles directly into the substantia nigra. They selected this approach over systemic administration because it provides precise targeting, avoids the blood–brain barrier and achieves a high local nanoparticle concentration with a low dose – potentially boosting treatment effectiveness.
Following injection of either nanoparticles or saline, the mice underwent pulsed NIR irradiation once a week for five weeks. The team then performed a series of tests to assess the animals’ motor abilities (after a week of training), comparing the performance of treated and untreated PFF mice, as well as healthy control mice. This included the rotarod test, which measures the time until the animal falls from a rotating rod that accelerates from 5 to 50 rpm over 5 min, and the pole test, which records the time for mice to crawl down a 75 cm-long pole.
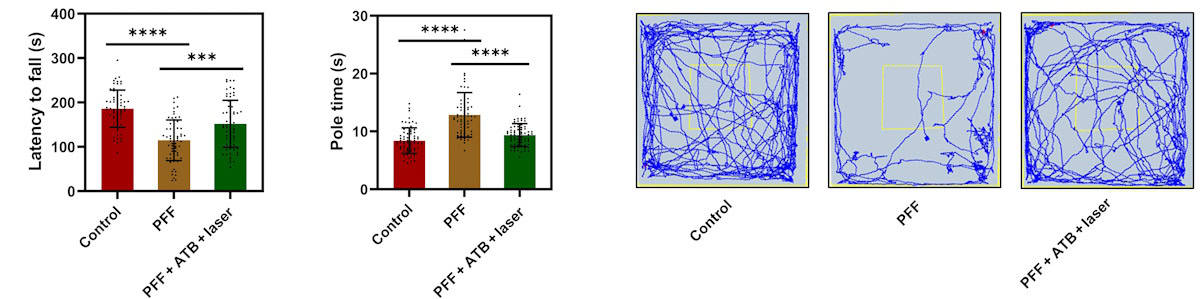
The team also performed an open field test to evaluate locomotive activity and exploratory behaviour. Here, mice are free to move around a 50 x 50 cm area, while their movement paths and the number of times they cross a central square are recorded. In all tests, mice treated with nanoparticles and irradiation significantly outperformed untreated controls, with near comparable performance to that of healthy mice.
Visualizing the dopamine neurons via immunohistochemistry revealed a reduction in neurons in PFF-treated mice compared with controls. This loss was reversed following nanoparticle treatment. Safety assessments determined that the treatment did not cause biochemical toxicity and that the heat generated by the NIR-irradiated ATB nanoparticles did not cause any considerable damage to the dopamine neurons.
Piezoelectric nanoparticles provide deep brain stimulation without invasive surgery
Eight weeks after treatment, none of the mice experienced any toxicities. The ATB nanoparticles remained stable in the substantia nigra, with only a few particles migrating to cerebrospinal fluid. The researchers also report that the particles did not migrate to the heart, liver, spleen, lung or kidney and were not found in blood, urine or faeces.
Chen tells Physics World that having discovered the neuroprotective properties of gold clusters in Parkinson’s disease models, the researchers are now investigating therapeutic strategies based on gold clusters. Their current research focuses on engineering multifunctional gold cluster nanocomposites capable of simultaneously targeting α-syn aggregation, mitigating oxidative stress and promoting dopamine neuron regeneration.
The study is reported in Science Advances.




