Deep brain stimulation (DBS), in which electrodes implanted in the brain deliver electrical impulses to specific targets, is an effective clinical treatment for several neurological conditions. DBS is currently used to treat movement disorders such as Parkinson’s disease, essential tremor and dystonia, as well as conditions such as epilepsy and obsessive-compulsive disorder. The treatment, however, necessitates brain surgery to insert the stimulation electrodes, with the potential to cause numerous side effects.
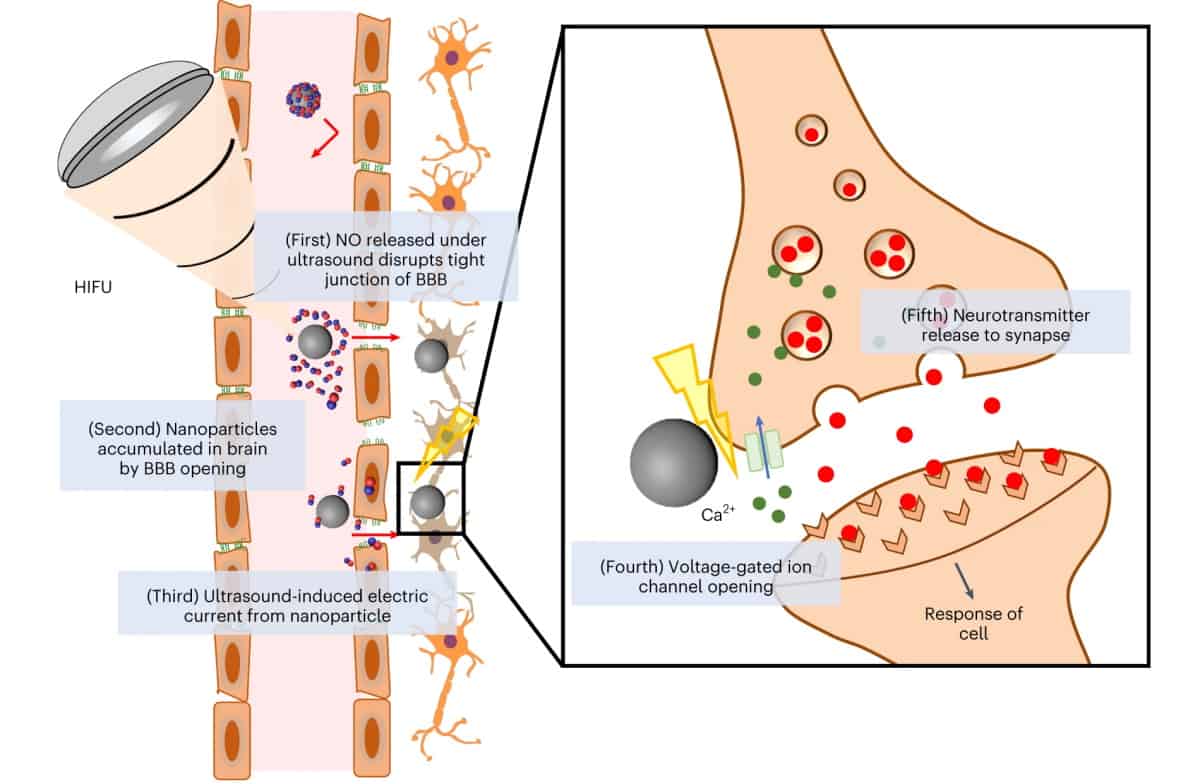
To remove the need for invasive surgery, researchers from Pohang University of Science and Technology (POSTECH) in Korea are developing a non-invasive neural stimulation strategy based on piezoelectric nanoparticles. The nanoparticles serve two functions – transient opening of the blood–brain barrier (BBB) and stimulating the release of dopamine – both controlled by externally applied focused ultrasound.
Piezoelectric nanoparticles are of interest as neural stimulators because in response to external stimuli – such as ultrasound, for example – they deform and output direct current. The researchers propose that this current could then be used to stimulate dopaminergic neurons to release neurotransmitters.
One key challenge is delivering the nanoparticles to the brain, specifically, how to get them across the BBB. To achieve this, the researchers turned to nitric oxide (NO), a highly reactive molecule that shows potential for BBB disruption. They designed a multifunctional system, described in Nature Biomedical Engineering, comprising a barium titanate nanoparticle coated with NO-releasing BNN6 and polydopamine (pDA). In response to ultrasound, these nanoparticles should generate both NO and direct current.
To test their approach, lead author Won Jong Kim and colleagues first investigated the nanoparticles’ ability to release NO. In response to 5 s of high-intensity focused ultrasound (HIFU), the nanoparticles instantaneously released NO. They also evaluated the piezoelectric behaviour using a patch-clamp set-up. While solvent without pDA-coated nanoparticles exhibited no current spikes, in the presence of the nanoparticles, distinctive current spikes were seen with intensities proportional to the ultrasound intensity.
DBS is hypothesized to electrically stimulate the nervous system by opening Ca2+ channels of nearby neurons and then accelerating neurotransmitter release at the synapse. To investigate whether nanoparticle-generated current could provide similar neural stimulation, the team monitored the Ca2+ dynamics of neuron-like cells. Intracellular Ca2+ concentration significantly increased in cells receiving both nanoparticles and ultrasound, whereas either ultrasound or nanoparticles alone did not have any effect.
Cells treated with ultrasound-stimulated nanoparticles also generated an increased extracellular concentration of dopamine, indicating Ca2+ influx-mediated neurotransmitter release. Again, no significant change was seen with either ultrasound or nanoparticles alone. Tests using non-piezoelectric nanoparticles showed insignificant changes in Ca2+ influx and neurotransmitter release, indicating that these effects arise primarily in response to piezoelectric stimulation.
The researchers next performed a series of in vivo studies. To investigate NO-mediated BBB opening, they intravenously injected mice with NO-releasing piezoelectric nanoparticles and then applied HIFU to targeted brain sites under ultrasound guidance.
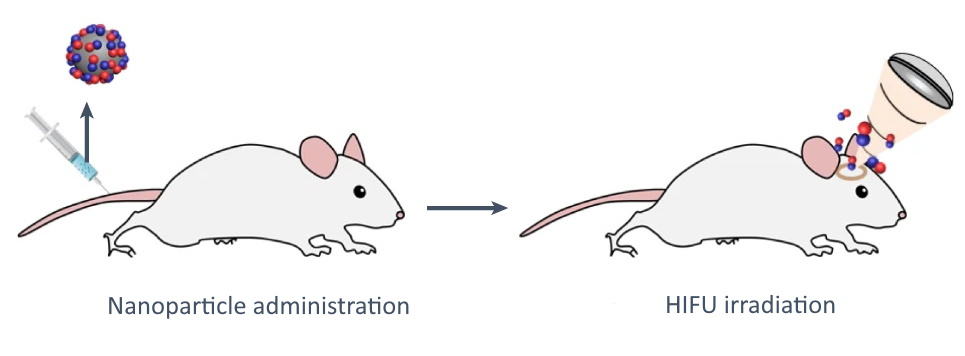
Two hours after injection, transmission electron microscopy revealed significantly higher amounts of nanoparticles accumulated inside the animals’ brains compared with control groups, demonstrating that the release of NO temporarily disrupted the tight junctions in the BBB. The researchers also showed that 2 h after HIFU application, the BBB was no longer permeable, confirming that the NO-mediated BBB disruption is only temporary.
Finally, the team evaluated the therapeutic effects of the nanoparticles using a mouse model of Parkinson’s disease. Mice were injected with nanoparticles followed by multiple applications of HIFU at the subthalamic nucleus (the US Food and Drug Administration-approved DBS targeting site) to restore dopamine levels in the brain.
DBS using the ultrasound-driven nanoparticles enhanced the animals’ behavioural functions, including motor coordination and locomotor activity. The mice showed a gradual improvement in motor function with daily HIFU stimulation for 10 days, with locomotor activity almost restored by day 16. The team surmise that the piezoelectric nanoparticles induced neurotransmitter release, which significantly alleviated the symptoms of Parkinson’s disease without causing any significant toxicity.

Personalized brain stimulation could treat untreatable depression
“We hope that ultrasound-responsive NO-releasing piezoelectric nanoparticles can be further developed into minimally invasive therapeutic approaches for the treatment of neurodegenerative diseases,” they conclude.
The group is now employing fundamental studies to determine out the underlying mechanisms for NO-mediated BBB opening. “We are also developing next-generation NO-modulatory materials to maximize their clinical usage while also minimizing their unwanted side effects,” explains first author Taejeong Kim.